SDAB Study Design and Biostatistics Core
Overview
The Biostatistics, Epidemiology, and Research Design (BERD) Core works closely with existing resources to provide targeted study design and biostatistics support to ITMAT/CTSA investigators. The Core serves as a direct provider of services, including protocol review, study design, proposal development, and the performance of simple to potentially substantial complex analyses, with potential to apply innovative cutting-edge methods developed by our faculty. BERD integrates the support available with the HUP and CHOP Center for Human Phenomic Science (CHPSs), the expertise and resources of faculty in the Department of Biostatistics, Epidemiology, and Informatics and Center for Clinical Epidemiology and Biostatistics (DBEI/CCEB), the Biostatistics Analysis Center (BAC), and the Biostatistics and Data Management Core (BDMC) at CHOP.
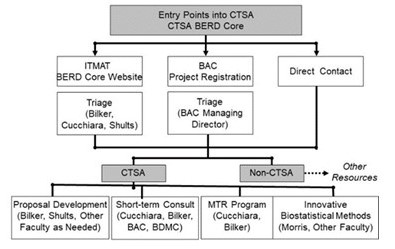
There are multiple routes to request assistance from members of the BERD Core as shown in the figure. The primary method is to send an email to sdab@mail.med.upenn.edu, which generates an email to Drs. Bilker, Cucchiara, and Shults. The second method is via the project registration process for the BAC; requests via that route are triaged and forwarded to Dr. Bilker if ITMAT-related. Direct contact to BERD personnel is also commonly used, especially for projects for which a previous collaboration exists. Regardless of the initial route of contact, all requests are reviewed and needs for faculty participation, design and/or data analysis assistance, and data management assessed. Any investigator requesting core support will need to be a registered ITMAT member and the proposed project will need to meet criteria of being a CTSA-appropriate project. Emphasis is placed on projects in accord with the two overarching themes of our CTSA – Translational Therapeutics and Bridging the Pediatric to Adult divide.
Contacts
BERD Core Director: Warren Bilker, PhD
Email: .warren@pennmedicine.upenn.edu
Adult: Andy Cucchiara, PhD
Email: andy@pennmedicine.upenn.edu
Pediatric: Abbas Jawad, PhD
Email: JAWAD@EMAIL.CHOP.EDU
Assistance in planning studies entails helping to define and refine the study question into testable hypotheses answerable by the available data, and then providing an appropriate study design that enables answering of this question. Design issues include identification of appropriate study endpoints, the type of design, sample size calculations to demonstrate feasibility and sufficient statistical power, implementation and logistical details, and cost. The quantitative background of core members equip them to help investigators focus and refine study hypotheses based on available data and their limitations. For example, if a proposed study design is not feasible in terms of cost, an experienced biostatistician and/or epidemiologist can help refocus the question or change the design, such as reducing the number of endpoints or measurements, while maintaining the goal of getting answers to relevant scientific hypotheses. Additionally, approaches such as blocked or randomized designs can be used to increase the probability of a representative sample and avoid confounding, contributing to assurance of reproducible and replicable research.
The study design assistance of the SDAB Core is also integrated with the Research Ethics Program Core (REP). For example, it is common for ethical considerations to be involved in the choice of study designs; a purely “optimal” study design in terms of statistical science may not be feasible due to ethical considerations regarding particular populations, such as pediatric studies, appropriateness of randomization, choices of control groups, or timing of informed consent. Members of the REC are available for the study design process, to provide expertise when the areas overlap.
The SDAB Core supports the investigators in making database and data management decisions and complying with regulatory issues relating to confidentiality and security of data. In this capacity, core members work directly with the ITMAT Center for Biomedical Informatics in Translation (BIIT). Appropriate data capture is crucial to the validity of not only to the overall integrity of a study, but also to statistical analysis. For example, many methods for longitudinal data analysis require repeated measurements on subjects to be in rows, rather than columns. Consultation on this type of issue at the design stage of a study can considerably reduce work required to transform datasets at the analysis stage. Core members consult with the entity that will manage the data and define clearly the statistical needs in order to later support the analysis of the data.
With the availability of REDCap, investigators are encouraged to use it or similar systems for analysis, rather than simple spreadsheets. Dr. Cucchiara serves as a consultant for investigators intending to use that resource. More complicated studies, or trials subject to a strong regulatory component, may require the use of Oracle Clinical. BAC members in the SDAB Core have extensive experience coordinating data management and analysis of data from Oracle Clinical in collaboration with BIIT, and will continue in the next period. These tasks include the overall data structure, data formats, creation of derived variables, and identification of potential data entry errors or outliers. Analysts may also be involved in the creation of validation rules at the database level, determining what types of checks and cross-form checks are needed in real time. These tasks outline an important collaboration with the BIIT, who also provide consultations in turn.
The primary goal of the statistical data analysis is to utilize analytical approaches to extract the information and knowledge embedded in the data and providing appropriate uncertainty measures, answering prospective hypotheses laid out in the specific aims and providing exploratory hypothesis-generating analyses of rich biomedical data. Methods employed range from the most basic descriptive statistics to state-of-the-art statistical methods that may involve multi-stage modeling, resampling methods, causal inference methods to adjust for potential biases in observational data, integrative models for multiple data types, predictive models for translational research, and innovative new methods developed by the world-class biostatistical faculty in our BERD. Specific areas of methodological expertise are described in the Innovative Biostatistical Methods page. Approaches to ensure reproducible research including multiple testing adjustment, proper model validation, post-selection inference, and rigorous documentation of analysis scripts will be employed, and care will be taken to ensure researchers understand the appropriate level of evidence and uncertainty of their results so that the strength of conclusions are clearly communicated in subsequent publications. More information on the latter item may be found under the Biostatistics and Epidemiology Program (BEMP).
At times, when they have sufficient training to do so and access to appropriate software, investigators may perform analyses themselves with guidance from BERD core members, especially investigators on training grants. Alternatively, a member of the BAC/DBMC, supported by the CTSA, is available to conduct limited analyses, with funding arrangements made for more extensive needs. This component of the BERD is crucial to the success of the core, both for investigators with limited expertise and/or resources, and for the application of more advanced and/or innovative methodology. Manuscript support is included as part of data analysis and includes composition of methods sections, production and/or review of tables and other statistical results presented, as well as interpretation of results and contribution to discussion sections of manuscripts as appropriate.