Program Overview
The Certificate Program provides training in translational science through the completion of four credit units of coursework and a research project. The certificate is designed for:
- PhD-level pre- or post-docs who want to extend their research into a clinical setting
- Pre- and post-graduate clinical trainees (MD, VMD, DMD, MSN) who want to enhance their expertise in clinical research to inform research at the bench
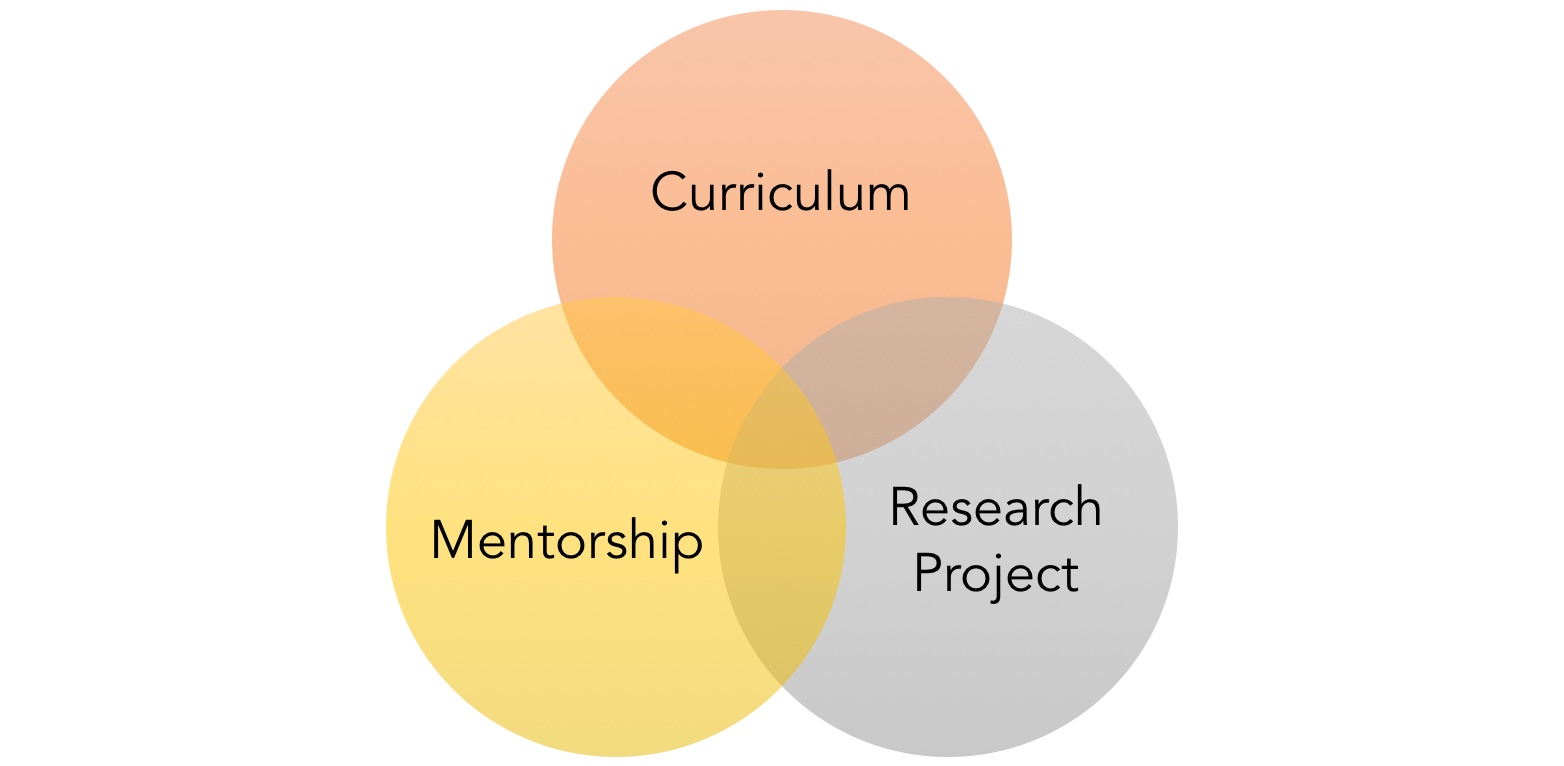
The program is built on three pillars: (1) a curriculum that focuses on regulatory science, clinical and translational science, or entrepreneurial science, (2) engagement in a one-year translational research project, and (3) active mentorship.
Curriculum Overview
The Certificate Program can be finished in one year and requires the completion of 4 credit units with a passing grade of B- or better and a research project. There are three certificates from which to choose:
- Certificate in Regulatory Science
- Certificate in Translational Science
- Certificate in Entrepreneurial Science
Faculty and Administration
Emma Meagher, MD, Program Director
Rachel McGarrigle, MSEd, Education Director
Megan Maxwell, MSW, Associate Director
Bethany Sanghani, MA, Associate Director
Jessica German, MSEd, Associate Director
Perelman School of Medicine | 8035 Maloney Building | 3400 Spruce Street | Philadelphia, PA 19104-4283 | 215-614-1835
There are three certificates from which to choose: the Certificate in Regulatory Science, the Certificate in Translational Science, or the Certificate in Entrepreneurial Science.
- The Certificate in Regulatory Science focuses on the development of new tools, standards, and approaches to assess the efficacy, quality, and performance of FDA-regulated products.
- The Certificate in Translational Science aims to develop a strong foundation in the fundamental techniques of translational research.
- The Certificate in Entrepreneurial Science focuses on the translation of biomedical research into innovative solutions and the development of approaches to commercialization.
Students choose at least 2 electives from their selected certificate based on their career goals. Students may take elective courses outside of these recommendations, but must obtain permission before enrolling.
The certificate programs require a one-year engagement in a research project. This will take the form of a new research project or as an additional aim to research currently being conducted. Prospective students will identify a mentor and define the research project at the time of application.
Tuition for the certificate is per course unit. Tuition rates and fees are listed on the Master’s Program Costs website. View Certificate program tuition costs by visiting the Master’s Program Costs website and scroll to the Master of Science in Translational Research section, click on the title to expand and view the tuition and fees.
Penn employees have access to tuition benefits, learn more about the Graduation Tuition Benefits and Tax.
Elective course units taken in the summer will be determined by the home school that offers that course.
Note: This program is not eligible for federal financial aid programs, but tuition benefits are available to qualifying faculty and staff.
Disclaimer
Please note that policies concerning admissions, curriculum, funding and financial aid are subject to change. Additionally, though variations in the curriculum may be possible, any changes will need prior approval and may have financial implications. This website is meant to provide preliminary general overview information only. Students interested in or enrolled in the program should seek personal advising from relevant faculty and staff.
Eligibility
Postdoctoral Applicants
- Entrants must have an MD, PhD, MSN, VMD, or DMD level degree.
Predoctoral Applicants
- Entrants must be students who are enrolled in a graduate degree program at Penn.
Application
The Online Certificate Application requires the following items:
Applicants, please add headers with your name to the applicable documents.
- Curriculum Vitae
- Personal statement: a one page description of career goals that includes a description of how the certificate meets your educational objectives
- Please note in your statement if you are applying for an ITMAT Funding award
- Transcripts
- Transcripts — ONLY for predoctoral applicants — upload an unofficial transcript from your current graduate program issued to you from your institution’s registrar
- If you are admitted to the program, official transcripts may be requested
If you are applying for ITMAT Funding, include:
- Research plan: 1-page description of proposed research project
- Entrepreneurial Certificate: project should emphasize innovation with the potential to commercialize
- Three letters of support from the applicant’s:
- Primary research mentor (send them the guidelines below)
- Secondary/co-mentor if applicable, or faculty member who can speak to your suitability for the Certificate program and/or experience in research
- Third faculty member who can speak to your suitability for the Certificate program and/or experience in research
- NOTE: Certificate applicants may only apply for TL1 awards and are not eligible for the ITMAT Scholarship
Creating a CollegeNet Account
- Create a CollegeNet account. After logging into CollegeNet, select “Online Application”.
- Enter your Personal Information. Save and continue to Program Information.
- Choose “Perelman School of Medicine Masters Programs”
- Select: “ITMAT Certificate Program”
- Select the earliest term you would like to begin the program.
- You will be asked to select your certificate concentration – Entrepreneurial, Regulatory, or Translational
- You can enter information in stages, at your own pace, and access the application any number of times until submission.
- Once the application has been fully submitted, check the status and note the receipt of recommendation letters.
Please note that the mentor's letter of support has specific guidelines
- The commitment to mentor the candidate
- The suitability of the trainee’s education objectives for stated career goals
- The feasibility and relevance of stated area of research to be undertaken during the program to include:
- The resources available to complete the research
- The availability of collaborative relationships that may be required to undertake the research question
- Mentor’s prior experience mentoring translational research trainees
- Add an appendix: Mentor’s biosketch and other support
Incomplete applications will not be considered.
We believe that a diverse clinical and translational science workforce will enable better science. Diverse teams of scientists bring an important range of experiences and perspectives that propel the collective potential for innovation. Thus, we seek to draw students from diverse backgrounds, including diversity of race, ethnicity, work and life experiences, interests, culture, socioeconomic status, sexual orientation, and those with disabilities.
Non-discrimination/Disability Policy
The University of Pennsylvania values diversity and seeks talented students, faculty and staff from diverse backgrounds. The University of Pennsylvania does not discriminate on the basis of race, color, sex, sexual orientation, gender identity, religion, creed, national or ethnic origin, citizenship status, age, disability, veteran status or any other legally protected class status in the administration of its admissions, financial aid, educational or athletic programs, or other University-administered programs or in its employment practices.
Questions or complaints regarding this policy should be directed to the Executive Director of the Office of Affirmative Action and Equal Opportunity Programs, Franklin Building, Suite 421, 3451 Walnut Street, Philadelphia, PA 19104-6205; or 215-898-6993 (Voice). Specific questions concerning the accommodation of students with disabilities should be directed to the Office of Student Disabilities Services located within Hamilton Village at 220 South 40th Street.
Jeanne Clery Disclosure of Campus Security Policy and Campus Crime Statistics Act
The federal Jeanne Clery Disclosure of Campus Security Policy and Campus Crime Statistics Act, as amended, requires colleges and universities to provide information related to security policies and procedures and specific statistics for criminal incidents, arrests, and disciplinary referrals to students and employees, and to make the information and statistics available to prospective students and employees upon request. The Campus SaVE Act of 2013 expanded these requirements to include information on and resources related to crimes of interpersonal violence, including dating violence, domestic violence, stalking and sexual assault. Federal law also requires institutions with on-campus housing to share an annual fire report with the campus community.
In addition, the Uniform Crime Reporting Act requires Pennsylvania colleges and universities to provide information related to security policies and procedures to students, employees and applicants; to provide certain crime statistics to students and employees; and to make those statistics available to applicants and prospective employees upon request.
To review the University’s most recent annual report containing this information, please visit: https://www.publicsafety.upenn.edu/clery/annual-security-fire-safety-report/ or http://www.upenn.edu/almanac/crimes-index.html.
You may request a paper copy of the report by calling the Office of the Vice President for Public Safety and Superintendent of Penn Police at 215-898-7515 or by emailing vp@publicsafety.upenn.edu
ITMAT Certificates Handbook
Useful Penn Links
- Canvas (course content management system)
- Penn Academic Calendar (actual course dates may vary from this calendar)
- Path@Penn (verify registration, billing, contact info)
- Tax Liability for Graduate Tuition Benefits
- Penn Policies and Resources for Graduate and Professional Students
Faculty and Administration
Emma Meagher, MD, Program Director
Rachel McGarrigle, MSEd, Education Director
Megan Maxwell, MSW, Associate Director
Bethany Sanghani, MA, Associate Director
Jessica German, MSEd, Associate Director
Perelman School of Medicine | 8035 Maloney Building | 3400 Spruce Street | Philadelphia, PA 19104-4283 | 215-614-1835
Certificate in Regulatory Science
The Certificate in Regulatory Science program is intended to meet the needs of the Penn Medicine community. This program is designed for PhD scientists who wish to pursue Regulatory Science careers in academia, the pharmaceutical & biotechnology industry, the consulting and legal industries, and federal agencies.
Pre-doctoral PhD students are encouraged to apply to the certificate and associated training grant. The CTSA TL1 award provides one year of funding including tuition and a stipend per NIH policy. The tuition funding will cover the cost of the certificate.
Coursework
The coursework provides students with training to build upon their scientific expertise and prepares them to understand regulatory strategy, the execution of clinical trials, and approaches to translate early discoveries and preclinical investigations into therapeutics. Students will learn the skills necessary to apply contemporary research methods to develop new tools, standards, and approaches to assess the efficacy, quality, and performance of FDA-regulated products.
Certificate in Regulatory Science Courses
| Required Courses | Electives |
|---|---|
|
|
The Certificate Program can be finished in one year and must be completed within two years. The certificate requires the completion of 4 credit units, with a passing grade of B- or better. Should a student wish to proceed onto the Master of Science in Regulatory Science the course work will count towards the degree.
If a student is interested in an elective that is not on the list, approval must be obtained from the Certificate Director.
Research Project
The certificate requires a one-year engagement in a regulatory science research project. This will take the form of a new research project or as an additional arm to research currently being conducted. If the student is already engaged in regulatory science an additional research project is not required. Prospective students will identify a mentor and define the research project at the time of application.
Certificate in Translational Science
Coursework
 The Certificate Program can be finished in one year and must be completed within two years. The certificate requires the completion of 4 credit units with a passing grade of B- or better. The certificate requires MTR 510 — Introduction to Clinical and Translational Research and MTR 600 — Introduction to Biostatistics. Other biostatistics courses may be substituted if taken in the past two years with a grade of B- or better. The remaining 2 credit units of coursework comprise electives from the certificate. Students may take courses outside of our MTR/REG offerings, but must obtain permission before enrolling.
The Certificate Program can be finished in one year and must be completed within two years. The certificate requires the completion of 4 credit units with a passing grade of B- or better. The certificate requires MTR 510 — Introduction to Clinical and Translational Research and MTR 600 — Introduction to Biostatistics. Other biostatistics courses may be substituted if taken in the past two years with a grade of B- or better. The remaining 2 credit units of coursework comprise electives from the certificate. Students may take courses outside of our MTR/REG offerings, but must obtain permission before enrolling.
Sample Study Plan
Certificate in Translational Science
Develop a strong foundation in the fundamental techniques of translational research.
- MTR 604 Scientific & Ethical Conduct
- MTR 620 Translational Therapeutics
- MTR 642 Building a Life Sciences Starup
- MTR 535 Intro to Bioinfomatics
- REG 618 Introduction to Vaccine Development
- REG 610 Fundamentals of FDA Regulation
- REG 611 Clinical Study Management
- REG 624 Applied Regulatory Processes of Vaccines and Biologics
- REG 612 Intro to Drug Development
- REG 613 Drug Development Decision Criteria
- REG 621 Cell & Gene Therapy
- REG 622 New Trends in Medicine and Vaccine Discovery
- REG 625 Challenges and Controversies in Academic Biomanufacturing
Research Project
The certificate requires a one-year engagement in clinical and translational research. This will take the form of a new research project or as a translational arm to research currently being conducted. Prospective students will identify a mentor and define the research project at the time of application
Certificate in Entrepreneurial Science
Coursework
 The Certificate Program in Entrepreneurial Science can be finished in one year and must be completed within two years. The certificate requires the completion of 4 credit units with a passing grade of B- or better. The certificate requires MTR 510 — Introduction to Clinical and Translational Research and MTR 600 — Introduction to Biostatistics. Other biostatistics courses may be substituted if taken in the past two years with a grade of B- or better. The remaining 2 credit units of coursework comprise electives from the chosen certificate. Students may take courses outside of our MTR/REG offerings, but must obtain permission before enrolling.
The Certificate Program in Entrepreneurial Science can be finished in one year and must be completed within two years. The certificate requires the completion of 4 credit units with a passing grade of B- or better. The certificate requires MTR 510 — Introduction to Clinical and Translational Research and MTR 600 — Introduction to Biostatistics. Other biostatistics courses may be substituted if taken in the past two years with a grade of B- or better. The remaining 2 credit units of coursework comprise electives from the chosen certificate. Students may take courses outside of our MTR/REG offerings, but must obtain permission before enrolling.
Sample Study Plan
Certificate in Entrepreneurial Science
Translate biomedical research into innovative solutions and develop approaches to commercialization
- MTR 640 Entrepreneurial Seminar
- HCMG 867 Healthcare Entrepreneurship
- MTR 620 Medical Entrepreneurship
- REG 610 Fundamentals of FDA Regulation
Students may take elective courses outside of these recommendations, by substituting a different MTR/REG course or course outside of our MTR/REG offerings, but must obtain permission before enrolling.
Research Project
The certificate requires a one-year engagement in clinical and translational research. This will take the form of a new research project or as a translational arm to research currently being conducted. Prospective students will identify a mentor and define the research project at the time of application.

