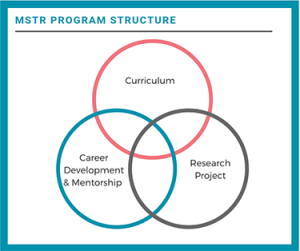
The objective of the Master of Science in Translational Research (MSTR) program is to provide students with in-depth instruction in the fundamental skills, methodology and principles necessary to become a well trained translational investigator. The program is designed to meet these objectives through:
- the provision of didactic course work,
- a formal mentorship program,
- formal laboratory training,
- a professional skills development program,
- and specific ongoing guidance with hands-on exposure to protocol and grant development.
Trainees are expected to complete a primary research project of their own design under the supervision of their primary mentor. The primary mentor will also play a role in helping the student identify a feasible research question for the thesis. The thesis should consolidate students’ knowledge of the principles and practice of translational research, and provide their first experience in writing a comprehensive NIH grant style proposal.
Upon successful completion of the MSTR program, graduates are expected to have developed a strong foundation in the fundamental techniques of translational research. They should be able to apply contemporary research tools to clinically relevant areas of investigation. The goal of the MSTR program is to produce clinical researchers who are competitive in seeking research support and who are knowledgeable about the complex issues associated with conducting sound translational research. The MSTR program will also assist in the promotion of translational research as a discipline within the Penn community.
Program Objectives
The primary objective is to produce a cadre of highly trained and sophisticated investigators adept in the skills necessary for the translational investigator; to prepare students for an academic career and to position them for future careers as successful academic researchers who will become leaders in their field of research interest.
Program Goals
The program is designed to meet these objectives though the provision of didactic in-depth instruction, a formal mentorship program, formal (wet or dry) laboratory training, and specific ongoing guidance with hands-on exposure to protocol and grant development. Individuals in this program are provided with the expertise and methods to attain their goals through learning the basic components of scientific training, the specific methods associated with their translational research interest, as well as training in biomedical research ethics and good clinical practice. MSTR students learn how to independently formulate meaningful hypotheses, design and conduct interpretable experiments, adhere to good laboratory and clinical practices, analyze results critically, understand the broad significance of their research findings, and uphold the highest ethical standards in research. The development of additional skills—including oral and written communication, grant writing, and laboratory management—are considered integral to this training.
Thesis
Students are required to engage in a research project of their own design under the supervision of the primary mentor. At the time of application, each student specifies the project they will pursue, along with the primary mentor who will supervise the research project. Students will use class material and homework assignments to assist in protocol development.
The research should be translational in nature and involve direct measurements on patient-derived samples or the use of innovative therapeutic or diagnostic techniques with laboratory-based elements. There should be demonstrable clinical relevance. The protocol is to be designed by the student under the direct supervision of the mentor. Where appropriate, dual mentorship should be considered; including a basic scientist expert in the technology being used and a clinical investigator expert in the condition being studied. The primary protocol should account for at least 75-80% of the student’s commitment to the program.
Trainees are expected to complete a thesis that involves designing a research project, writing a formal research proposal, performing the study described in it, preparing 1-2 comprehensive scholarly scientific paper(s) reporting the results, and presenting and defending the thesis at a public seminar. The defense portion of the seminar will be a formal oral defense of the thesis with three examiners.
Mentoring Program
An essential component of the MSTR degree program is the mentoring program. Effective mentoring is critical not only for research training but also to allow the trainee to develop into an independent investigator. Read more here.
Learn more about the mentor and mentee experience:
Administration
Emma A. Meagher, M.D., Program Director
Perelman School of Medicine
8032 Maloney Building
3400 Spruce Street
Philadelphia, PA 19104-4283
Telephone: 215-662-2174
emma@upenn.edu
ITMAT Education Staff
Rachel McGarrigle, MSEd, Education Director
Megan Maxwell, MSW, Associate Director
Jessica German, MSEd, Associate Director
Danielle Williams, Education Coordinator
Perelman School of Medicine
8033 Maloney Building
3400 Spruce Street
Philadelphia, PA 19104-4283
Telephone: 215-614-1835
itmated@pennmedicine.upenn.edu
Overview
MSTR Curriculum
The MSTR degree is composed of 12 course units, under 4 pillar areas
- Research Methods & Implementation (3 c.u. required)
- MTR 6020 Proposal Development and Study Design (1 c.u.)
- MTR 9999 Lab units (2 c.u.)
- Analytical Skills (1 c.u. required)
- Responsible Conduct of Research (1 c.u. required)
- MTR 6040 Scientific and Ethical Conduct
- Scientific Writing (1 c.u. required)
- MTR 6010 Scientific Writing I
- MTR 6050 Scientific Writing II
- MTR 6060 Grantsmanship (Career Development Award)
- MTR 6230 Writing an NIH Grant
- Remaining 4 c.u. guided by MSTR concentration and/or research interests
- Successful MSTR thesis (MSTR 6070 (1 c.u.), MSTR 6080 (1 c.u.)
- View Sample Study Plan
Core Courses
- MTR 6000: Introductory Biostatistics (1.0 c.u.)
- MTR 6010: Review Writing (1.0 c.u.)
- MTR 6020: Proposal Development (1.0 c.u.)
- MTR 6030: Disease Measurement (1.0 c.u.)
- MTR 6040: Scientific and Ethical Conduct (1.0 c.u.)
- MTR 6050: Manuscript Writing (1.0 c.u.)
Description of Core Courses
Required Core Courses
MTR 6000 Introductory Biostatistics: (Fall – year one)
This course approaches statistics from an applied as well as theoretical point of view. Students learn the correct application and interpretation of basic statistical concepts and techniques. The course covers probability estimation, hypothesis testing, nonparametric tests, tests for categorical data, correlation, and regression. Students will be provided with an understanding of statistical methods, skills in the use of software to apply those methods and the critical thinking to interpret analytic results produced by your effort and/or that of fellow researchers.
MTR 6010 Scientific Writing I: Review Writing (Summer II – year one)
This course will lead students through the process of writing a Review Article during their first Summer within the MSTR program. Review articles will be authored with the student’s primary mentor and will be used to accomplish the following goals:
- Attain rapid familiarity with background in their new area of study;
- A mechanism for mentor and student to create a productive working/writing relationship;
- Help the student identify key gaps in the literature and/or areas of controversy that would benefit from pivotal experiments;
- Understand the factors that contribute to variability in research outcomes in their area and;
- Introduce the student to other scientists in their new area through an initial publication early in their career.
Mentors will be asked to agree to participate in this process, or identify another senior individual in their group who would perform the function as a condition to have MSTR students funded in their program. The course director and members of the curriculum committee will provide guidance and critical reviews throughout the process.
MTR 6020 Proposal Development and Study Design: (Summer II through Fall – year one)
This course focuses on study design and proposal development as they relate to the studies that probe the mechanism of disease. It discusses concepts such as writing a background section, asking a research question, designing a study, use of biomarkers, writing a research proposal, overview of different study designs and addressing feasibility issues. Development of the thesis proposal starts during this course and concludes with each student submitting and presenting their proposal to the MSTR faculty panel for critique and feedback.
MTR 6030 Disease Measurement Course: (Fall – year one)
Acquire the knowledge to rationally and effectively incorporate disease measurements, including emerging technologies, into the design of translational and clinical research protocols. Gain a basic understanding of measurement methodologies used in clinical medicine. Understand how “normal” values are determined, and how to interpret test results in the context of patients/research subjects. Approach disease measurements (tests) as a mean of answering questions, and to be able to choose appropriate tests to answer the questions being posed. The measurement aspects of the students’ research protocol are written and evaluated during this course.
MTR 6040 Scientific and Ethical Conduct: (Spring – year one)
In this course, students will learn the foundational principles of scientific and ethical conduct of research, complete directed experience in evaluating these principles through IRB membership and ultimately be able to apply them to their own work. By the end of the foundational class sessions, students will understand scientific conduct, ethical considerations including human subjects and animal protections, regulations governing the use of health information, drugs, and devices, good laboratory practices, conflict of interest, and ethics in challenging new research domains. The directed experience will include membership for six months on an Institutional Review Board (IRB) at either the University of Pennsylvania or the Children’s Hospital of Philadelphia. This membership experience will expose students to real issues, considerations, and solutions in human subjects research and study design.
MTR 6050 Scientific Writing II – Manuscript Writing: (Summer I – year two)
Students will write a primary data manuscript for publication with their primary lab mentor. Emphasis will be placed on identifying publishable data that was either generated by the student, or which is made available to the student for analysis from the mentor’s lab (e.g. perform a new analysis across data from multiple studies, organize and analyze data that is ‘laying in wait’ for someone to publish it). The student will be expected to learn the role of first author including:
- coordination with the senior mentor to write the introduction,
- organize data, analyses and figures;
- obtain or write methods and results from collaborators;
- writing a discussion and;
- “getting it out the door”.
The authorship for the publication is left to the discretion of the mentor in consultation with the originator of the data and the MSTR student. This will both teach the student the value of publishing as an integral part of academic life, and will facilitate their success with subsequent grant applications. The course director will provide guidance and critical review of work throughout the process. Mentors will be asked to agree to participate in this process, or identify another senior individual in their group who would perform the function. Completion of the course and continuation of associated funding is contingent on submission of the manuscript.
Electives
Students enroll in elective courses based on their area of Concentration.
Description of Electives
Students must take electives that total two credit units. Electives must be graduate level courses that complement the student’s project and future career plans in translational research. Electives require prior approval of the MSTR Mentoring Committee.
A sample of elective suggestions are listed by Concentration.
ITMATEd courses (MSTR and REG) that may be considered for electives can be found under ITMAT Education Courses.
Elective courses outside of the Perelman School of Medicine can also be considered.
Laboratory Units
- MTR 9999 (1.0 c.u.)
- MTR 9999 (1.0 c.u.)
Description of Laboratory Units
MSTR degree candidates are required to complete two lab units of primary, meaningful laboratory research in a translational research setting. Successful completion of each lab rotation results in the awarding of one credit unit. The purpose of the lab rotation is to emphasize the basic components of the translational research experience, to appreciate that the underpinnings of translational research is understanding disease mechanism, and to learn the subtleties of the measurement of disease process and the complexity this brings to the area of human research.
The student formulates a lab proposal, conducts the research in the laboratory, collects data, and analyzes it. Each lab rotation is meant to provide experience working in a new environment or learning a new technique. Examples of lab units include, but are not limited to:
- a traditional wet bench experience to learn how to develop an assay
- a clinical lab rotation learning how to perform and analyze a technique in your specialty
- a rotation in a bioinformatics laboratory
- a rotation in an imaging laboratory
Optional: Industry Internship
The MSTR program, in collaboration with its corporate partners, will provide an opportunity for students per semester to learn about translational medicine in a Pharmaceutical Industry Internship. This may be eligible for a MSTR 999 lab unit.
Description of Industry Internship
Industry Internship Program: The MSTR program, in collaboration with its corporate partners, will provide an opportunity for interested students to learn about translational medicine in a Pharmaceutical Industry Internship. The internship will include approximately 10 hours per week for one semester. Internships may span across various facets of the pharmaceutical industry, including discovery, development, regulatory affairs and/or commercialization. Students will gain hands on experience “translating technology” and will receive credit for the internship. This program will foster greater interactions between industry and academia by exposing MSTR students to the roles they can play in the pharmaceutical industry as a potential career path. Students will be expected to work on site at the corporate partners location for 1 day per week, with additional time dedicated to background research and preparation.
Evaluation process: Students will have both a university and corporate mentor that will participate in training and evaluation. Nalaka Gooneratne will serve as the university mentor for all MSTR industry interns. Corporate mentors will be assigned based on the specific content and department in which the internship is performed. Mentors will work together to ensure that interns are meeting the goals and expectations of the internship and the MTR 999 course requirements.
Eligibility: All students enrolled in the MSTR program are eligible to participate in the internship. An agreement exists between industrial partners and UPenn that permits this educational experience. The components of this agreement, which will be explained to students, include completing a free standing company application form that includes both a background check and drug testing.
Thesis
- MTR 6070: Thesis (1 c.u.)
- MTR 6080: Thesis (1 c.u.)
Description of Thesis
Students are required to engage in a research project of their own design under the supervision of the primary mentor. At the time of application, each student specifies the project they will pursue, along with the primary mentor who will supervise the research project. Students will use class material and homework assignments to assist in proposal development.
The research should be translational in nature and involve direct measurements on patient-derived samples or the use of innovative therapeutic or diagnostic techniques with laboratory-based elements. There should be demonstrable clinical relevance. The protocol is to be designed by the student under the direct supervision of the mentor. Where appropriate, dual mentorship should be considered; including a basic scientist expert in the technology being used and a clinical investigator expert in the condition being studied. The primary protocol should account for at least 75-80% of the student’s commitment to the program.
Trainees are expected to complete a thesis that involves designing a research project, writing a formal research proposal, performing the study described in it, preparing 1-2 comprehensive scholarly scientific paper(s) reporting the results, and presenting and defending the thesis at a public seminar. The defense portion of the seminar will be a formal oral defense of the thesis with three examiners.
Disclaimer
Please note that policies concerning admissions, curriculum, funding and financial aid are subject to change. Additionally, though variations in the curriculum may be possible, any changes will need prior approval and may have financial implications. This website is meant to provide preliminary general overview information only. Students interested in or enrolled in the program should seek personal advising from relevant faculty and staff.
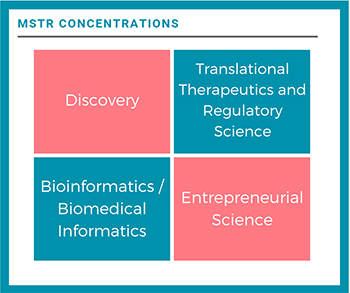
Discovery
Discovery is for trainees who are elucidating the basic pathophysiological etiology and/or process of disease. Projects may be proof of concept in cell or animal models or human samples.
Curriculum
Degree Requirements (12 credit units):
MTR Core Courses
MTR 6000 Introduction to Biostatistics
MTR 6010 Review Writing (with an emphasis on discovery)
MTR 6020 Proposal Development
MTR 6030 Disease Measurement
MTR 6040 Scientific and Ethical Conduct
MTR 6050 Manuscript Writing
Labs
MTR 9999-001 Lab (with an emphasis on discovery)
MTR 9999-002 Lab (with an emphasis on discovery)
Thesis
MTR 6070 Thesis (with an emphasis on discovery)
MTR 6080 Thesis (with an emphasis on discovery)
Suggested Electives (Choose 2 c.u.)
- MTR 6060 Grantsmanship or MTR 6230 Writing an NIH Grant
- BIOM 555 Regulation of the Genome
- CAMB 510 Immunology
- CAMB 512 Cancer Genetics and Biology
- CAMB 609 Vaccines and Immune Therapeutics
- CAMB 630 Topics in Human Genetics and Disease
- CAMB 632 Cell Control by Signal Transduction Pathways
- CAMB/GCB 752 Genomics
- BMB 585 Wistar Advanced Cancer Biology Course: Cancer Pathways
- PHRM 564 Drug Delivery
- PHRM 570 Principles of Cardiovascular Biology: Vascular biology, medicine and engineering
- PHRM 580: Topics in Pharmacogenomics
Translational Therapeutics and Regulatory Science
Translational Therapeutics and Regulatory Science focuses on testing discoveries for preclinical and/or clinical effects. Projects may be first in humans, phase 1, or assessing the safety, efficacy, quality, and performance of regulated products.
Curriculum
Degree Requirements (12 credit units):
Core Courses
- MTR 6000: Introductory Biostatistics (1.0 c.u.)
- MTR 6010: Review Writing (1.0 c.u.)
- MTR 6020: Proposal Development (1.0 c.u.)
- MTR 6030: Disease Measurement (1.0 c.u.)
- MTR 6040: Scientific and Ethical Conduct (1.0 c.u.)
- MTR 6050: Manuscript Writing (1.0 c.u.)
Two Laboratory Units
MTR 9999-001: Lab (with an emphasis in translational therapeutics)
MTR 9999-002: Lab (with an emphasis in translational therapeutics)
One Elective
Thesis
- MTR 6070: Thesis (with an emphasis in translational therapeutics)
- MTR 6080: Thesis (with an emphasis in translational therapeutics)
Suggested Electives
- MTR 6060 Grantsmanship or MTR 623 Writing an NIH Grant
- MTR 6210 Cell and Gene Therapy
- REG 6220 New Trends in Medicine and Vaccine Discovery
- REG 6100 Fundamentals of FDA Regulation
- REG 6110 Clinical Study Management
- REG 6120 Intro to Drug Development
- REG 6140 Biopharmaceutical Development, Manufacturing, and Regulatory Affairs
- REG 6300 Clinical Trials
- EPID 6380 Topics in Clinical Trial Design & Analysis
Entrepreneurial Science
The MSTR Concentration in Entrepreneurial Science is designed for students who aim to navigate both business and academic environments. This concentration offers trainees the opportunity to translate biomedical research into innovative solutions and to develop approaches to commercialization. Graduates of the program are expected to have a more robust entrepreneurial mindset coupled with tangible skills to bring biomedical research to market. The program is designed to support a trainee as they acquire skills in key aspects of: 1) Needs assessment, 2) Idea development, 3) Scientific methodology, and 4) Approaches to commercialization.
Entrepreneurial Science Concentration Director: Nalaka Gooneratne, MD, MSc
Curriculum
Courses
| Term | Courses |
|---|---|
| Summer Year 1 | MTR 6020: Proposal Development
MTR 6010: Review Writing (or EntSci elective for postdocs) |
| Fall Year 1 | MTR 6000: Introductory Biostatistics
MTR 6030: Disease Measurement MTR 6400: Entrepreneurial Science Seminar (Part I) |
| Spring Year 1 | MTR 6040: Scientific and Ethical Conduct
HCMG 867: Healthcare Entrepreneurship |
| Summer Year 2 | MTR 6050: Data Manuscript Writing
MTR 9999: Lab |
| Fall Year 2 | EntSci Elective
MTR 9999: Lab |
| Spring Year 2 | MTR 6070: Thesis Credit MTR 6080: Thesis Credit (including commercialization plan) |
Required Entrepreneurial Science Courses
MTR 640 Entrepreneurial Science Seminar: This course reviews a broad range of topics related to establishing a successful career as an academic entrepreneur. The goal is to help students successfully navigate both business and academic environments as they conduct research on their concept, and consider commercialization opportunities.
HCMG 867 Healthcare Entrepreneurship: The goal of the course is to give students the hands-on experience of establishing and operating an early-stage healthcare or life sciences business by, among other things, working as part of a mentored group to craft and defend a business plan based on an actual technology or service in the space (defined as therapeutics, diagnostics, medical devices, or digital health services).
Suggested Electives
- MTR 6060 Grantsmanship or MTR 623 Writing an NIH Grant
- MTR 6240 SBIR/STTR Grants (In Dev)
- MTR 6200 Medical Entrepreneurship
- HCIN 607-001 Translating Ideas into Outcomes
- HCMG 853 Medical Devices
- HCMG 863 Management and Economics of the Pharmaceutical & Biotech Industries
- HCMG 866 E-Health: Business Models and Impact
- MGMT 712 Managing Strategic Partnerships
- MGMT 892 Collaborative Innovation Program
- NURS 357 Case Study: Innovation in Health: Foundations of Design Thinking
- EAS 545 Engineering Entrepreneurship I
- EAS 546 Engineering Entrepreneurship II
Business Mentorship
MSTR-EntSci student mentoring will mirror that for traditional MSTR students with a key inclusion of a business mentor. At the time of matriculation into the MSTR, a mentorship team is formed for each student. The composition of the team is determined by the research project and is typically composed of the primary research mentor, a secondary mentor, a biostatistics mentor, a MSTR program mentor, and a business mentor. This team serves as an ongoing monitoring group for the student’s progress. Its members are faculty with expertise relevant to both the basic and clinical aspects of the candidate’s research and each is expected to contribute their expertise to fostering the candidate’s research progress. The business mentor will provide their expertise to develop the mentee’s ability to effectively communicate his/her scientific ideas in a business context and navigate the field. An additional area of focus for the mentoring team is to cultivate the student’s skills to be an effective member of a research team and impart approaches to leading a research team.
Business Mentorship
The business mentor is a unique addition to the traditional MSTR Mentor Committee. The business mentor will participate in the mentoring committee meetings throughout the duration of the program and hold periodic meetings with the student individually. The role of the business mentor is to guide, train, advise, and promote the career development of the student. This may include:
- Provide business related expertise
- Work to develop the mentee’s ability to effectively communicate his/her scientific ideas in a business context
- Assist the mentee in identifying opportunities and avoiding threats to his/her future success (i.e. IP considerations, market analysis, cost-benefit analysis)
- Support the mentee’s development of confidence and expertise to navigate within the business realm
- Help identify learning opportunities and key steps tailored to the mentee’s goal (i.e. funding, project development, internships, collaborations, identifying skills and gaps)
Entrepreneurial Ecosystem
ITMATEd is building a community to support trainees with entrepreneurial goals. This consists of resources, team mentoring, a network of entrepreneurial peers and faculty, and the robust Penn ecosystem.
Academic Entrepreneurship Blog and E-Book
The Academic Entrepreneurship Blog was expanded and published into an e-book, Academic Entrepreneurship for Medical and Health Scientists, which serves as a repository of tools, wisdom and best practice that seeks to democratize innovation regardless of where the academic researcher resides. There are more than 50 chapters of material, divided across the five major sections: Academia, People, Ideation, IP/Reg, and Finance.
Innovation Mixers
Innovation Mixers are designed to facilitate multi-disciplinary team building relevant to life sciences entrepreneurship and training. The ITMAT Education program has piloted the development of a monthly networking event in partnership with multiple other Schools (Wharton Business, Law, Design, Engineering, Education, and Dental). As part of the registration process for Innovation Mixers attendees indicate their expertise and specific domains in which they require assistance. A software application (InnoMix) developed for the Innovation Mixers then pairs individuals in a series of four to six meetings (analogous to speed dating) held at the Mixer, each lasting 15 minutes, thus providing attendees with a unique opportunity to meet others who could help them fill gaps in their team.
On Campus Education, Resources, and Activities related to Entrepreneurial Science
University
Penn Medicine/PSOM
Wharton
- MBA Entrepreneurship Management
- MBA Health Care Management
- Wharton Venture Initiation Program
- Penn Biotech Group Healthcare Consulting
- Wharton Small Business Development Center
- Penn Wharton Commercialization Workshop
Engineering
Law
- Detkin IP and Technology Legal Clinic
- Entrepreneurship Legal Clinic
- Penn Law Center for Technology, Innovation and Competition
Design
PCI
Undergraduates
External Opportunities
- The Science Center’s QED Proof-of-Concept Program seeks QED Fellows with an interest in the life sciences and related technologies to assist in developing commercially focused projects at selected universities and other research organizations in the Greater Philadelphia Region. The QED Program provides mentorship and funding to R&D projects to demonstrate the commercial feasibility of early-stage life science technologies. Working under the direction of QED Business Advisors (industry professionals and entrepreneurs) and with program personnel, research scientists, and Technology Transfer Offices, QED Fellows will help guide the development of R&D proposals for projects that are designed to retire business risk, thereby enabling follow-on commercialization through licensing or investment in new companies. Candidates will apply for the QED Fellow role through a formal application process.
- Chestnut Street Ventures, a venture fund composed of Penn alumni investing together into Penn alumni-led companies, has created a Venture Fellow Program to provide Penn alumni with the opportunity to gain increased exposure to the world of venture capital. The program allows participants to experience the entire lifecycle of a venture fund: raising capital, investor relations, deal sourcing, diligence, and making investments. No prior experience in venture capital is required and there is no financial cost to those who are selected to join the program. In order to apply, applicants must complete a formal submission process through the fellow portal.
- Additional industry internships are available.
MSTR-EntSci Sample Projects
Caroline Gluck, M.D.
CHOP Nephrology Fellow
Primary Research Mentor: Katalin Susztak, M.D., Ph.D.
Project Title: Do Epigenetic Changes in Patients with Minimal Change Disease Predict Steroid Responsiveness?
Project Description: Epigenetics refers to changes in gene expression patterns that are not caused by alterations in the nucleotide sequence itself. This project will investigate epigenetic changes that occur in patients with minimal change disease (nephrotic syndrome), aiming to use this information to develop diagnostic reagents that will predict steroid responsiveness.
Lindsey George, M.D.
CHOP Hematology Instructor
Primary Research Mentor: Rodney Camire, Ph.D.
Project Title: Zymogen-like Factor Xa Variant as Novel Warfarin Reversal Strategy: Pre-clinical Evaluation and Mechanism of Action
Project Description: Warfarin is the most commonly used anticoagulant and is very effective at preventing blood clots, but is associated with bleeding side effects that can be fatal. Currently there are no medications that reliably improve outcome when these bleeding events occur. This project is studying a recently developed molecule called Factor XaI16L that may prove to be more effective at reversing warfarin and may provide insight into how blood clots are formed.
Amelia Keaton, M.D.
CHOP Infectious Diseases Fellow
Primary Research Mentor: David Weiner, Ph.D.
Project Title: Development of a Synthetic DNA Vaccine Against Influenza
Project Description: Despite worldwide vaccination efforts, the influenza virus remains a significant cause of illness and death. Constant genetic change requires that new vaccines be created each season for influenza, yet current vaccines fail to induce immunity against a wide variety of influenza strains. The goal of this project is to develop an alternative influenza vaccine that uses synthetic DNA technology to protect patients from a wide range of influenza viruses.
Helge Hartung, M.D.
CHOP Hematology Assistant Professor
Primary Research Mentor: Mortimer Poncz, M.D.
Project Title: Development of an Automated Bone Marrow Device in Order to Improve the Quality of Diagnostic Bone Marrow Aspirates and Biopsies, Decrease Procedure Times, and Improve Clinical Outcomes for Patients with Leukemia and Other Blood Disorders
Project Description: More than 50% of all childhood cancers require a bone marrow evaluation as part of their work-up. To perform a bone marrow evaluation, bone marrow aspiration and biopsy needles are pushed or drilled into the marrow space by the physician performing the procedure. The goal of this project is to improve this procedure by developing an automated penetration device that allows the user to get better specimens faster, resulting in fewer adverse effects for the patient and cost savings for the public.
Amanda Ackermann, M.D., Ph.D.
CHOP Endocrinology and Diabetes Instructor
Primary Research Mentor: Klaus Kaestner, Ph.D.
Project Title: Generating a Human Beta Cell Line
Project Description: Diabetes is caused by insufficient insulin produced by pancreatic beta cells, and beta cell transplantation is a promising therapy. Unfortunately, human beta cells are in short supply and have been very difficult to culture in vitro. The goal of this project is to generate a new self-propagating and functional human beta cell line from primary pancreatic tissue surgically resected from patients undergoing pancreatectomy for hyperinsulinism.
Máire Abraham Conrad, M.D., M.S.
CHOP Gastroenterology Instructor
Primary Research Mentor: Marcella Devoto, Ph.D.
Project Title: Characterization of Intestinal Microbiome in Very Early Onset InflammatoryBowel Disease (VEO-IBD)
Project Description: Inflammatory bowel disease diagnosed in children less than 5 years old is a distinct, rare yet growing entity known as very early onset inflammatory bowel disease (VEO-IBD), with strong genomic contributors, and occasionally monogenic gene defects,that confer a heterogeneous IBD phenotype. The rapidly increasing incidence and prevalence of VEO-IBD supports the hypothesis that the environmental factors and the microbiome contribute to this phenotype. This project aims to describe the microbiome of patients with VEO-IBD and assess the interaction between host genetics with taxonomic dysbiosis in order to provide the groundwork for creating diagnostic testing to identify dysbiosis specific to certain immune defects associated with VEO-IBD.
Brian Jenssen, M.D., M.S.H.P.
CHOP General Pediatrics Instructor
Primary Research Mentor: Alexander Fiks, M.D., M.S.C.E.
Project Title: Clinical Decision Support Tool for Parental Tobacco Treatment in Primary Care
Project Description: Secondhand smoke (SHS) exposure is a significant public health problem in that it both harms children and is widely prevalent, affecting more than 40% of US children. Pediatricians are uniquely positioned to educate and motivate parents towards protecting their children from SHS, and health information systems, such as electronic health records (EHRs) and clinical decision support (CDS) systems, can improve the quality and standardization of clinical interventions for tobacco use. This project expands upon pilot work to further refine and more rigorously evaluate a tobacco treatment CDS tool integrated within the EHR to increase delivery of smoking cessation treatment to parents in the context of their child’s medical visit.
Ari Wes, B.A.
Perelman School of Medicine M.D. Candidate
Primary Research Mentor: Jesse Taylor, M.D.
Project Title: An Assessment of Forces in Craniofacial Distraction Osteogenesis and the Development of a Novel Distraction System
Project Description: Distraction represents a critical tool in the craniofacial armamentarium, but it has significant limitations that prevent its widespread adoption. One of the most profound limitations, and a feature seen in all currently available distractors, is the external component that protrudes through the patient’s skins to allow for manual engagement of the device. The goal of this project is to gain a better of understanding of the forces required throughout the distraction period to inform the development of a novel, fully implantable distraction system.
Bioinformatics/Biomedical Informatics
The rapidly emerging field of biomedical informatics defines how we compare and evaluate health data to both understand and introduce improvements to care (medical informatics), as well as the use of health data to conduct discovery-based investigation of biological systems (bioinformatics).
A Bioinformatics/ Biomedical Informatics concentration in the MSTR degree program was designed to introduce students to informatics and to provide a framework through which data science skills can be utilized in their research. Thus, we focus on the following objectives:
- Introduce students to informatics and its use in research
- Develop knowledge to facilitate productive interactions and/or collaboration with expert informatics specialists
- Develop a capability around a specific computational tool or analysis pipeline
- Develop a capability of rudimentary programming and data processing
The concentration is appropriate for students who would like to develop a specific type of computational skill set or experimental pipeline to analyze data they have generated, as well as students who are not sure what particular data type or set they will utilize in the future, and instead aim to develop general computational skills and approaches to augment their research objectives. Indeed, no prior programming or data science skills are required to select this track.
Bioinformatics / Biomedical Informatics Concentration Director: Ben Voight, PhD
Curriculum
Degree Requirements (12 credit units):
MTR Core Courses
MTR 6000 Introduction to Biostatistics
MTR 6010 Review Writing (with an emphasis on Bioinformatics)
MTR 6020 Proposal Development
MTR 6030 Disease Measurement
MTR 6040 Scientific and Ethical Conduct
MTR 6050 Manuscript Writing
Labs
MTR 9999-001 Lab (with an emphasis on Bioinformatics)
MTR 9999-002 Lab (with an emphasis on Bioinformatics)
Thesis
MTR 6070 Thesis (with an emphasis on Bioinformatics)
MTR 6080 Thesis (with an emphasis on Bioinformatics)
Required Courses
MTR/CIS/GCB 5350 Introduction to Bioinformatics or
EPID 6000/BMIN 5030 Data Science for Biomedical Informatics
One (1) Advanced Elective in Data Science/Statistics
Suggested Electives (Choose 1 c.u.)
- BMIN 5010 Introduction to Biomedical and Health Informatics
- BMIN 5210 Ai II: Machine Learning
- BMIN 5250 Introduction to Python Programming
- STAT 5710 Modern Data Mining
- CAMB 7140 DIYtranscriptomics
- BSTA 7870 Methods for Statistical Genetics and Genomics in Complex Human Disease
- GCB 5330 Statistics for Genomics and Biomedical Informatics
Tuition rates and fees are listed on the Master’s Program Costs website. Tuition is listed per course unit and students are required to take 2 course units per term. View the MSTR program tuition costs by visiting the Master’s Program Costs website and scroll to the Master of Science in Translational Science section, click on the title to expand and view the tuition and fees.
The MSTR degree has a total of 12 course units. Tuition and fees for elective course units taken in the summer will be determined by the home school that offers that course.
Penn employees have access to tuition benefits, learn more about the Graduation Tuition Benefits and Tax.
Predoctoral Financial Information
Billing for MD Students
MD-MSTR students will be charged 7 semesters of medical tuition (6 semesters in years 1-3 and the final semester of year 5). During Fall and Spring semesters of year 4 and the Fall semester of year 5, MSTR tuition will be charged per course unit.
Students take 2 or 3 courses during the semesters they are charged medical school tuition. MTR 607 & 608 are taken in final semester of year 5 (see Sample Study Plan). As long as the student has not already used the benefit that allows med students to take three courses while registered for the MD program, there will be no additional charge beyond MD tuition in those semesters.
For most students, the tuition charges for the MSTR will be 3 courses per term during Fall and Spring of year 4 and the Fall semester of year 5. Tuition for these 9 units will total approximately $44,076. MD-MSTR students will be billed for 7 semesters of MD tuition, rather than 8. If the student is grant funded ($20,500 toward tuition), and is eligible for the PSOM additional course policy, this will leave approximately $3,368 to be paid by the student. (If a student has already used their eligibility for the 3 additional courses per term, there will be additional charges for MTR 607 and 608 in the final semester).
As soon as a student is considering the pursuit of an integrated joint degree program, financial aid planning counseling and planning is strongly advised. Students should contact the School of Medicine Office of Admissions and Financial Aid. Staff members are available to help explain program costs, the financial aid process, funding options and how to apply for financial aid.
Please refer to the Combined Degree website for more information.
Billing for Dental and Nursing Students
Predoctoral students enrolled in DMD or MSN/PhD programs should contact their financial aid office to discuss the financial implications of enrolling in the MSTR degree program.
Grant Funding
A potential partial funding mechanism for predoctoral students in the MSTR program is the Clinical and Translational Science Award (CTSA) training grant. Selected students will be appointed to the grant for 12 months with a TL1 award. Learn more about the CTSA TL1.
Disclaimer
Please note that policies concerning admissions, curriculum, funding and financial aid are subject to change. Additionally, though variations in the curriculum may be possible, any changes will need prior approval and may have financial implications. This website is meant to provide preliminary general overview information only. Students interested in or enrolled in the program should seek personal advising from relevant faculty and staff.
Eligibility
Postdoctoral Applicants
- Entrants must have MD, PhD, MSN, VMD, or DMD level degree
- Entrants must enroll as full-time students and are not eligible to apply unless 80% of their time is protected
Predoctoral Applicants
- Applicants must be enrolled at the University of Pennsylvania in the MD, DMD, VMD, Nursing, or Biomedical Science PhD programs.
- MD-MSTR Applications are due in the fall of year 3 of the MD program.
- DMD-MSTR Applications are due in the fall of year 2 of the DMD program and follow the dental dual-degree application process.
Application Support
ITMAT Education Faculty and Administration are glad to connect with interested applicants throughout the year. Through an initial consult, program faculty or staff can gain understanding to which program may best align with your career goals, connect to current students or faculty for additional insight, answer questions about the application process, and provide feedback on project proposals.
Contact us and a member of the team will reach out to set a time to discuss your interests.
Application
The Online MSTR Application requires the following items:
Applicants, please add headers with your name to the applicable documents.
- Curriculum Vitae
- Personal statement (one page)
- Describe your career goals and what skills you need to reach those goals
- Include a learning plan of the activities that will help you attain these skills
- Predoctoral (MD, DMD, VMD) Students, please address the following:
- What motivated you to pursue the MSTR program versus the other Master Degree programs or ‘year out’ research opportunities?
- Why do you want to pursue formal research training during graduate school versus a later stage of training?
- Why are you seeking formal training in Translational Research?
- Research plan: 2-3 page description of proposed research project
- *Entrepreneurial track: project should emphasize innovation with the potential to commercialize
- External support: description of the applicant’s support awarded or under review for salary, tuition, or research supplies
- Please be as specific as possible when describing current or potential support: e.g. appointed to a T32 training grant, dates of appointment, amount available for tuition support each year.
- Please specify if you prefer to be considered for a specific funding mechanism: TL1, KL2, ITMAT Scholarship
- If you do not have other support, please indicate this in the document.
- Primary Mentor NIH biosketch and other support
- Transcripts — ONLY for predoctoral applicants — upload an unofficial transcript from your current graduate program issued to you from your school’s registrar
- Three letters of support from the applicant’s:
- Primary mentor (send them the guidelines below)
- Secondary/co-mentor if applicable, or faculty member who can speak to your suitability for the MSTR program and/or experience in research
- Third faculty member who can speak to your suitability for the MSTR program and/or experience in research
- For postdoctoral applicants, choose another faculty member
- For all predoctoral applicants, choose another faculty member from undergraduate or graduate/medical school
- Application Fee: $45 by credit card via CollegeNet
Creating a CollegeNet Account
- Create a CollegeNet account. After logging into CollegeNet, select “Online Application”.
- Enter your Personal Information. Save and continue to Program Information.
- Choose “Perelman School of Medicine Masters Programs”
- Post-doctoral and Faculty MSTR Applicants select: “Translational Research, MS, Post-doctoral applicants”
- Pre-doctoral MSTR Applicants select: “Translational Research, MS, Pre-doctoral applicants”
- Select “Summer 2023” term.
- You can enter information in stages, at your own pace, and access the application any number of times until submission.
- Once the application has been fully submitted, check the status and note the receipt of recommendation letters.
Please note that the letters of support have specific guidelines:
Primary Mentor Letter Guidelines
The Mentor(s) letter of support should address the following:
- The commitment to mentor the candidate
- The suitability of the trainee’s education objectives for stated career goals
- The feasibility and relevance of stated area of research to be undertaken during the program to include:
- The resources available to complete the research
- The availability of collaborative relationships that may be required to undertake the research question
- Mentor’s prior experience mentoring in ITMAT programs and/or mentoring translational research trainees
*Entrepreneurial track: include relevant experience as an innovator - Add an appendix: Mentor’s biosketch and other support
Applications received after the deadline or incomplete applications will not be considered.
We believe that a diverse clinical and translational science workforce will enable better science. Diverse teams of scientists bring an important range of experiences and perspectives that propel the collective potential for innovation. Thus, we seek to draw students from diverse backgrounds, including diversity of race, ethnicity, work and life experiences, interests, culture, socioeconomic status, sexual orientation, and those with disabilities.
Non-discrimination/Disability Policy
The University of Pennsylvania values diversity and seeks talented students, faculty and staff from diverse backgrounds. The University of Pennsylvania does not discriminate on the basis of race, color, sex, sexual orientation, gender identity, religion, creed, national or ethnic origin, citizenship status, age, disability, veteran status or any other legally protected class status in the administration of its admissions, financial aid, educational or athletic programs, or other University-administered programs or in its employment practices.
Questions or complaints regarding this policy should be directed to the Executive Director of the Office of Affirmative Action and Equal Opportunity Programs, Franklin Building, Suite 421, 3451 Walnut Street, Philadelphia, PA 19104-6205; or 215-898-6993 (Voice). Specific questions concerning the accommodation of students with disabilities should be directed to the Office of Student Disabilities Services located within Hamilton Village at 220 South 40th Street.
Jeanne Clery Disclosure of Campus Security Policy and Campus Crime Statistics Act
The federal Jeanne Clery Disclosure of Campus Security Policy and Campus Crime Statistics Act, as amended, requires colleges and universities to provide information related to security policies and procedures and specific statistics for criminal incidents, arrests, and disciplinary referrals to students and employees, and to make the information and statistics available to prospective students and employees upon request. The Campus SaVE Act of 2013 expanded these requirements to include information on and resources related to crimes of interpersonal violence, including dating violence, domestic violence, stalking and sexual assault. Federal law also requires institutions with on-campus housing to share an annual fire report with the campus community.
In addition, the Uniform Crime Reporting Act requires Pennsylvania colleges and universities to provide information related to security policies and procedures to students, employees and applicants; to provide certain crime statistics to students and employees; and to make those statistics available to applicants and prospective employees upon request.
To review the University’s most recent annual report containing this information, please visit: https://www.publicsafety.upenn.edu/clery/annual-security-fire-safety-report/ or http://www.upenn.edu/almanac/crimes-index.html.
You may request a paper copy of the report by calling the Office of the Vice President for Public Safety and Superintendent of Penn Police at 215-898-7515 or by emailing vp@publicsafety.upenn.edu.
COVID-19 Pandemic: Spring 2020
Recognizing the challenges of teaching, learning, and assessing academic performance during the global COVID-19 pandemic, Penn’s admissions committees for graduate and professional programs will take the significant disruptions of the COVID-19 outbreak in 2020 into account when reviewing students’ transcripts and other admissions materials as part of their regular practice of performing individualized, holistic reviews of each applicant. In particular, as we review applications now and in the future, we will respect decisions regarding the adoption of Pass/Fail and other grading options during the period of COVID-19 disruptions. An applicant will not be adversely affected in the admissions process if their academic institution implemented a mandatory pass/fail (or similar) system for the term or if the applicant chose to participate in an optional pass/fail (or similar) system for the term. Penn’s longstanding commitment remains to admit graduate and professional student cohorts composed of outstanding individuals who demonstrate the resilience and aptitude to succeed in their academic pursuits.

Adam Cuker, MD, MSTR
MSTR Graduate, 2010
Current position: Assistant Professor of Pathology and Laboratory Medicine, University of Pennsylvania Perelman School of Medicine
Adam enrolled in the MSTR program because “the course work was practical and applicable to (his) research. At the same time, meeting the requirements for graduation proved not to be an undue burden on (his) time and, in fact, increased the efficiency and methodologic rigor with which (he) was able to pursue (his) research.” Throughout the program, Adam “was made aware of a number of critical resources for investigators throughout the institution that (he has) subsequently used for (his) own research.” Adam identified the MSTR degree as a valuable credential for grant applications and job searches.
Adam completed the degree while he was a Fellow in Hematology/Oncology at the Hospital of the University of Pennsylvania. After graduation, Adam was awarded a K23 grant from the NHLBI for his study, “Improving the Diagnosis of Heparin‐induced Thrombocytopenial.”
Check out Adam’s publications on Pub Med.

Nuala Meyer, MD, MSTR
MSTR Graduate, 2011
Current position: Assistant Professor of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pennsylvania Perelman School of Medicine
Before enrolling in the degree program, Nuala was in a basic science lab but aspired to do large-scale genomic studies in human cohorts. She desired deeper training in clinical research, genetics, and informatics. For Nuala, the “MSTR offered a flexible, personalized course of study to help (her) acquire specific skills helpful to (her) research, and simultaneously a comprehensive overview of clinical trial design, human subject protection, statistical methodology, and novel lab techniques. (She) found it quite invaluable to development as a translational researcher.”
Nuala found the mentorship component of the program “supremely important” and helped her make contacts across the Penn community who are now her collaborators.
Nuala “would definitely recommend (the MSTR degree program) to anyone interested in pursuing translational research, with a balanced approach to both the human population and to the molecular or imaging methodologies used. The program is best suited to someone with a clear idea of his/her research questions at the outset, and with a focused need for specific coursework.”
Nuala completed the degree while she was an Instructor in Pulmonary, Allergy, and Critical Care Medicine at the Hospital of the University of Pennsylvania. After graduating, she was awarded a K23 from the NHLBI for her study, “Genetic Variation in the ANGPT-TIE Pathway and Risk for Acute Lung Injury.”
Check out Nuala’s publications on PubMed.

Nathan Singh, MD, MSTR
MD-MSTR Graduate, 2013
Current position: Internal Medicine Resident, Hospital of the University of Pennsylvania
Nathan enrolled in the MSTR because he “wanted to really learn what translational research looks like – how it is structured and how physician-scientists can bridge this gap.” Nathan found that the “landmarks set by the program, including proposal development and presentation, grant submission and thesis defense all forced (him) to continually move forward and re-evaluate progress in the lab, and provided an opportunity to receive feedback from experienced faculty.” He described the feedback process as his “own private study section!”
Nathan was a combined degree student and completed the MSTR degree in conjuction with medical school. While enrolled, Nathan was able to attend several professional conferences, give a talk at one of these conferences, and have exposure to leaders in the field. He described the MSTR degree as helpful in residency interviews and residency placement. Even at an early stage in his career, he has submitted several small foundation grants and attributes part of this success to his MSTR education.
Check out Nathan’s publications on PubMed.
MSTR Documents:
- Student Handbook
- MSTR Mentor Compact
- MSTR FAQs
Useful Links:
- Canvas (course content management system)
- Penn Academic Calendar (actual course dates may vary from this calendar)
- Penn In Touch (verify registration, billing, contact info)
- Tax Liability for Graduate Tuition Benefits
- Penn Policies and Resources for Graduate and Professional Students
- Penn Handbook on Ethics and Original Research
- Edge for Scholars — Candid commentary, gritty truths, sharpen your academic edge
Additional Program Support:
- Citing ITMAT Education Funding Awards (KL2, TL1, ITMAT Scholarship)
- KL2/TL1/ITMAT Scholar Finances: Lorri Schieri and Andrea Albelda
- MD-MSTR Combined Degree: Program Office
2023 Defenses
2023 Thesis Defense Schedule
- MSTR Thesis Defense: Tristan Lim Thesis title: Early Inflammation and Interferon Signaling Coordinate Enhanced Intestinal Crypt Wednesday, April 5th, 2023 @ 1:30pm 8031 Maloney Building, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA, 19104
- MSTR Thesis Defense: Joanna Wang, MD Loss and accelerated senescence of alveolar epithelial type II cells in pulmonary fibrosis in a murine model of Hermansky-Pudlak syndrome Monday, April 17th, 2023 @ 3:00pm 8030 Maloney Building, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA, 19104
- MSTR Thesis Defense: Debanjan Haldar Thesis Title: “Application of Unsupervised Machine Learning in Radiogenomic Classification of Pediatric Low Grade Gliomas” Tuesday, April 25th, 2023 @ 3:00pm Virtual
- MSTR Thesis Defense: Garrett Santini Thesis title: “Deciphering Heterogeneity Amongst Lamina-Associated Domains (LADs) in Development and Disease” Monday, May 1st, 2023 @ 2:00pm Smilow Center for Translational Research, 3400 Civic Center Boulevard, Philadelphia, PA 19104
- MSTR Thesis Defense: Kelly Sloane, MD Transcranial Direct Current Stimulation to Ameliorate Impairments in Neurocognition After Stroke Monday, May 1st, 2023 @ 2:00pm Virtual
- MSTR Thesis Defense: Christina Murphy Thesis Title: “Wound scabs containing neutrophil extracellular traps promote fibrotic skin repair” Wednesday, May 3rd, 2023 @ 9:00am Virtual
- MSTR Thesis Defense: Jelte Kelchtermans, MD Thesis Title: “Ambient air pollution sensitivity in pediatric asthma” Monday, May 8th, 2023 @ 10:00am Virtual
- MSTR Thesis Defense: Austin Borja Human neuronal dynamics and state of consciousness Wednesday, May 10th, 2023 @ 10:00am Virtual
- MSTR Thesis Defense: Bryce Perler, MD The Roleof the Colon in the Effect of Diet on the Human Plasma and Gut Metabolome Wednesday, May 24th, 2023 @ 10:00am Virtual
2022 Defenses
2022 Thesis Defense Schedule
- MSTR Thesis Defense: Sandra P. Susanibar-Adaniya, MD “Sox2 T-cell receptor engineered therapy for cancer therapy. A detailed characterization of Sox2 CD8+ T-cell immunity in plasma cell disorders” Tuesday, January 11th, 2022 @ 10:00am Virtual
- MSTR Thesis Defense: Jessica Breton, MD Dietary inflammatory potential and food patterns in relation to gut microbiome among children with Crohn’s disease Thursday, March 17th, 2022 @ 1:00pm Virtual
- MSTR Thesis Defense: Sarah Gitto, PhD Overcoming Resistance to Standard of Care Treatments for Ovarian Cancer.” Monday, April 18th, 2022 @ 1:30pm Virtual
- MSTR Thesis Defense: Kobie Mensah-Brown Cerebral Organoids as a Platform for Neuromodulation Monday, April 25th, 2022 @ 4:30pm Virtual
- MSTR Thesis Defense: James ‘Jimmy’ Germi Use of electrophysiologic recordings and behavioral experiments to study the efficacy of therapeutic interventions for traumatic brain injury in rodent models Wednesday, May 4th, 2022 @ 3:00pm Virtual
- MSTR Thesis Defense: Heather Giannini, MD Transcriptomic Profiling in Sepsis: Interrogating Gene Expression for Mechanistic Discovery and Therapeutic Targets in Critical Illness Syndromes Thursday, June 23rd, 2022 @ 1:00pm Virtual
- MSRS Thesis Defense: Lisa Ann Varughese, PharmD CLINICAL IMPLEMENTATION OF DPYD AND UGT1A1 PHARMACOGENETIC TESTING AT PENN MEDICINE Tuesday, June 28th, 2022 @ 12:00pm Virtual
2021 Defenses
2021 Thesis Defense Schedule
2020 Defenses
2020 Thesis Defense Schedule
- MSTR Thesis Defense: Leah R. Zuroff, BA Inflammatory Mechanisms in the Aging MS Patient Wednesday, April 15th, 2020 @ 11:00am Bluejeans Web Conference
- MSTR Thesis Defense: Laura Adang, MD, PhD Neurologic Outcomes in AGS Wednesday, April 22nd, 2020 @ 2:00pm Bluejeans Web Conference
- MSTR Thesis Defense: Abhi Ramachandran “Cardiac Microtissue Platform with Dynamically Tunable Afterload Control” Tuesday, May 5th, 2020 @ 10:00am Bluejeans WebConference
- MSTR Thesis Defense: Carissa Livingston “Sunitinib-Induced Cardiotoxicity in an Engineered Cardiac Microtissue Model” Wednesday, May 6th, 2020 @ 1:30pm Bluejeans Web conference
- MSTR Thesis Defense: Kevin McNerney, MD “Invariant Natural Killer Cell Immunotherapy in Neuroblastoma” Wednesday, May 6th, 2020 @ 3:30pm Bluejeans Web Conference
- MSTR Thesis Defense: Alexandra Dreyfuss “A novel mouse model to study image-guided radiation-induced cardiac injury and identify clinically relevant predictors of toxicity” Thursday, May 7th, 2020 @ 9:30am Bluejeans Web conference
- MSTR Thesis Defense: Suzanne MacFarland, MD “Identification of Novel Genomic Drivers of Juvenile Polyposis Syndrome” Thursday, May 7th, 2020 @ 10:00am Bluejeans Web Conference
- MSTR Thesis Defense: Steve S. Cho, BS “Evaluation of Diagnostic Accuracy Following the Coadministration of Delta-Aminolevulinic-Acid and Second-Window Indocyanine-Green in Rodent and Human Glioblastomas” Thursday, May 7th, 2020 @ 11:00am Bluejeans WebConference
- MSTR Thesis Defense: Drew Goldberg, BA “A Comparative Analysis of the Cardioprotective Effects of Mesenchymal Stem Cell Extracellular Vesicles Versus Endothelial Progenitor Cells” Friday, May 8th, 2020 @ 8:00am Bluejeans WebConference
- MSTR Thesis Defense: Courtney Quinlan, DO The Genetics of Pediatric Obstructive Sleep Apnea Friday, May 29th, 2020 @ 12:00pm Zoom WebConference
- MSTR Thesis Defense: Melanie Ruffner, MD, PhD “Innate and Adaptive Immune Mechanisms as Targets for Translational Therapy in Eosinophilic Esophagitis” Tuesday, June 2nd, 2020 @ 1:00pm Bluejeans Web Conference
- MSTR Thesis Defense: Elisia Clark, PhD “Tissue Engineered Nigrostriatal Pathway as a Testbed for Evaluating Axonal Pathophysiology in Parkinson’s Disease” Friday, June 12th, 2020 @ 1:30pm Blue Jeans Web Conference
- MSTR Thesis Defense: Laura Ferguson, MD MSTR Thesis Defense Tuesday, June 16th, 2020 @ 3:00pm Zoom WebConference
- MSTR Thesis Defense: Lauren N. Krumeich, MD “Pathologic Profiling of T-lymphocytes in Hepatocellular Carcinoma” Thursday, June 18th, 2020 @ 2:00pm Zoom WebConference
- MSTR Thesis Defense: Paige Meizlik, MD “The Thyroid Axis in Older Individuals with Persistent Subclinical Hypothyroidism” Friday, June 26th, 2020 @ 2:00pm Zoom WebConference
- MSTR Thesis Defense: Austin Pantel, MD “PET Imaging of Glutamine Metabolism with [18F] (2S,4R)4-Fluoroglutamine: Kinetic Analysis and Human Translation” Thursday, July 30th, 2020 @ 1:00pm Bluejeans
2019 Defenses
2019 Thesis Defense Schedule
- MTR Thesis Defense: Jeffrey Thompson, MD Development of circulating tumor biomarkers for the management of non-small cell lung cancer Wednesday, January 16th, 2019 @ 2:30pm 9025 Maloney Building,HUP, 3400 Spruce Street, Philadelphia, PA 19104
- MSTR Thesis Defense: Anthony R. Martin, BSE Nanofibrous Acellular Hyaluronic Acid Scaffold with Embedded TGF-β3 and SDF-1α for Articular Cartilage Repair: Translation into a Large Animal Model Wednesday, April 3rd, 2019 @ 1:00pm 316 Stemmler Building, 3450 Hamilton Walk, Philadelphia, PA 19104
- MSTR Thesis Defense: Neil N. Patel, BA, BS The Role of Solitary Chemosensory Cells in Promoting Type-2 Inflammation of the Upper Airway Tuesday, April 9th, 2019 @ 2:30pm 8030 Maloney Building, HUP, 3400 Spruce St., Philadelphia, PA 19104
- MSTR Thesis Defense: Robert Schwab, BA Chimeric Antigen Receptor T Cell Therapy for Atherosclerosis Monday, April 15th, 2019 @ 9:00am Room 8-146, Smilow Center for Translational Research, 3400 Civic Center Boulevard, Philadelphia, PA 19104
- MSTR Thesis Defense: Alex Morrison, BA Agonistic CD40 combines with immune checkpoint blockade to reverse T cell exhaustion and induce long term cures in murine pancreatic adenocarcinoma Monday, April 22nd, 2019 @ 1:00pm SCTR 12-146 AB, Smilow Center for Translational Research, 3400 Civic Center Boulevard, Philadelphia, PA 19104
- MSTR Thesis Defense: Elliot Stein, BA “Development of a percutaneous electrolytic ablation device and observation of tissue death in vitro with real-time magnetic resonance imaging” Wednesday, April 24th, 2019 @ 1:30pm 506EW Jordan Medical Education Center, 3400 Civic Center Boulevard, Philadelphia, PA 19104
- MSTR Thesis Defense: Chris Corbett, BA Assessment of a Novel Folate Receptor Alpha-Targeted Dye for Intraoperative Molecular Imaging in Lung Cancer Thursday, April 25th, 2019 @ 2:00pm 8031 Maloney Building, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA, 19104
- MSTR Thesis Defense: Nicolas Seranio, BS Elucidation of the role of circulating tumor cells in the management of bladder cancer Monday, April 29th, 2019 @ 11:30am 8030 Maloney Building, HUP, 3400 Spruce Street, Philadelphia, PA
- MSTR Thesis Defense: Yohannes Ghenbot, BS Learning Active Sensing Strategies Using a Sensory Brain Machine Interface Tuesday, April 30th, 2019 @ 2:30pm 9025 Maloney Building, HUP, 3400 Spruce Street, Philadelphia, PA
- MSTR Thesis Defense: Laura Vella, MD, PhD Cell States in the Thoracic Duct: Circulatory Intermediates of T Follicular Helper Cells between Lymph Nodes and Blood Monday, May 6th, 2019 @ 1:00pm 9025 Maloney Building, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA, 19104
- MSTR Thesis Defense: Alexandra Marquez, MD Brain resuscitative oxygen strategies in pediatric cardiac arrest Monday, May 6th, 2019 @ 1:30pm 8030 Maloney Building, HUP, 3400 Spruce St., Philadelphia, PA 19104
- MSTR Thesis Defense: John D. Arena, BA Tau Phenotyping of Chronic Traumatic Encephalopathy Recapitulates Aging-Related Pathologies Tuesday, May 14th, 2019 @ 12:30pm 8030 Maloney Building, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA, 19104
- MSTR Thesis Defense: Julia D’Souza, BSE Development of Antivascular Ultrasound (AVUS) Therapy for Hepatocellular Carcinoma in a Rat Model Tuesday, May 14th, 2019 @ 1:30pm 8031 Maloney Building, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA, 19104
- MSTR Thesis Defense: Victoria Gershuni, MD, MS “The interaction between fat, fiber, and the intestinal microbiome is communicated to host via bile acid signaling” Thursday, May 30th, 2019 @ 9:30am BRB 253, Biomedical Research Building, 421 Curie Blvd. Philadelphia, PA 19104
- MSTR Thesis Defense: Rosaline Zhang, BA Bone-Selective MRI as a Non-Radiative Alternative to CT for Craniofacial Imaging Friday, May 31st, 2019 @ 8:00am 8030 Maloney Building,HUP, 3400 Spruce Street, Philadelphia, PA 19104
- MSTR Thesis Defense: Anupam Kumar, MD Use of noninvasive imaging techniques for assessment of cardiovascular hemodynamics and risk of death or readmission in heart failure Tuesday, June 11th, 2019 @ 8:00am 9025 Maloney Building, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA, 19104
- MSTR Thesis Defense: David Coughlin, MD Molecular Endotypes of Lewy Body Disorders Tuesday, June 11th, 2019 @ 3:00pm 9025 Maloney Building, HUP, 3600 Spruce Street, Philadelphia, PA
- MSTR Thesis Defense: Hilary Faust, MD Role of Mitochondrial DNA in the Pathogenesis of Organ Failure in Critical Illness Friday, June 21st, 2019 @ 9:00am 8030 Maloney Building, HUP, 3600 Spruce Street, Philadelphia, PA
- MSTR Thesis Defense: Constantine Mavroudis, MD “Advanced Neuromonitoring in a Swine Model of Cardiopulmonary Bypass and Deep Hypothermic Circulatory Arrest” Thursday, September 26th, 2019 @ 1:00pm 8031 Maloney Building, Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA, 19104
- MSTR Thesis Defense: Brian Jenssen, MD, MSHP “Parent eReferral to Tobacco Quitline: A Pragmatic Randomized Trial in Pediatric Primary Care” Thursday, November 7th, 2019 @ 10:30am 10-110 Roberts Center for Pediatric Research
- MSTR Thesis Defense: Eliot Peyster, MD “Computational Histologic Analysis for the Diagnosis of Heart Transplant Rejection” Friday, November 22nd, 2019 @ 1:30pm SCTR 1-102, Smilow Center for Translational Research, 3400 Civic Center Boulevard, Philadelphia, PA 19104
2018 Defenses
2018 Thesis Defense Schedule
- MTR Thesis Defense: Catherine Norise “Post-Stroke and Neurodegenerative Aphasias: Noninvasive Neuromodulation Technologies and Predictive CSF Analytes” Thursday, February 22nd, 2018 @ 2:00pm 8029 Maloney Building, HUP, 3600 Spruce Street, Philadelphia, PA 19104
- MTR Thesis Defense: Caroline Gluck, MD “Identification of Novel Pathways in Chronic Kidney Disease Progression through Epigenome Wide Changes in Human Kidney Tubules” Monday, March 5th, 2018 @ 2:30pm 9025 Maloney Building, HUP, ITMAT Classroom
- MTR Thesis Defense: Ramani Balu, MD, PhD Friday, March 9th, 2018 @ 10:00am 8030 Maloney Building, HUP, 3600 Spruce Street, Philadlephia, PA 19104
- MTR Thesis Defense: Ari M. Wes ”Novel Considerations in Craniomaxillofacial Distraction” Friday, April 27th, 2018 @ 3:00pm 8030 Maloney Building (8th Floor), 3600 Spruce Street, Philadelphia, PA 19104
- MTR Thesis Defense: Thomas F. Tropea, MD “Biomarkers as predictors of cognitive decline in Parkinsons Disease” Tuesday, May 1st, 2018 @ 12:00pm Room 505, Jordan Medical Education Center, 3400 Civic Center Boulevard, Philadelphia, PA 19104
- MTR Thesis Defense: Nishat Shahabuddin “Autophagy in response to Aggregatibacter actinomycetemcomitans and LtxA” Wednesday, May 2nd, 2018 @ 10:00am Levy Building, 3rd Floor Conference Room, Penn Dental School, 4010 Locust Street, Philadelphia, PA, 19104
- MTR Thesis Defense: Arka Mallela “Finding the Stealth Pathology of Mild Traumatic Brain Injury” Thursday, May 3rd, 2018 @ 12:00pm 9025 Maloney Building (9th Floor), 3600 Spruce Street, Philadelphia, PA 19104
- MTR Thesis Defense: Nina A. Ran “Single B Cell Receptor Profiling to Investigate Tolerance Checkpoints in Pemphigus Vulgaris” Tuesday, May 8th, 2018 @ 9:00am 9025 Maloney Building (9th Floor), 3600 Spruce Street, Philadelphia, PA 19104
- MTR Thesis Defense: Chantae Sullivan-Pyke, MD “Gonadotropin Stimulation has Differential Effects on Oocyte and Embryo Development: Evidence from a Mouse Model” Wednesday, July 25th, 2018 @ 12:00pm BRB 253, Biomedical Research Building, 421 Curie Blvd. Philadelphia, PA 19104
- MTR Thesis Defense: Deepthi Alapati, MD In utero gene editing for monogenic lung disease Wednesday, November 14th, 2018 @ 10:00am 11 – 100, Smilow Center for Translational Research, 3400 Civic Center Boulevard, Philadelphia, PA 19104
2017 Defenses
2017 Thesis Defense Schedule
- MTR Thesis Defense: Greg Nadolski, MD “Near Infrared Fluorescence Imaging of Matrix-Metalloprotease-2 Activity as a Biomarker of Vascular Remodeling in Hemodialysis Access “ Wednesday, February 22nd, 2017 @ 1:00pm 8030 Maloney, ITMAT Classroom, 3600 Spruce Street, Phildadelphia, PA 19104
- MTR Thesis Defense: George W. Fryhofer IV, AB “Post-Injury Biomechanics of Achilles Tendon Vary by Sex and Hormone Status” Tuesday, February 28th, 2017 @ 1:30pm 8030 Maloney Building, ITMAT Classroom, HUP
- MTR Thesis Defense: Harry J. Han, BS “The Role of ACVR1 in Pediatric Diffuse Intrinsic Pontine Gliomas: Insights from the Bone” Monday, April 24th, 2017 @ 3:30pm 8031 Maloney Bldg, 3600 Spruce Street, Philadelphia, PA 19104
- MTR Thesis Defense: Delu Song, MD, PHD “Retinal analysis in mice and patients treated with systemic iron: a potential risk for age-related macular degeneration” Wednesday, April 26th, 2017 @ 1:00pm 8030 Maloney Builidng, HUP
- MTR Thesis Defense: Jennie Lin, MD “Transcriptome-Wide Analyses of Human Cellular Models of Inflammation” Wednesday, June 14th, 2017 @ 2:30pm 9025 Maloney Building, HUP
- MTR Thesis Defense: Amanda Muir, MD “Influence of age and eosinophilic esophagitis on esophageal distensibility in a pediatric cohort” Thursday, July 13th, 2017 @ 9:00am 9025 Maloney Building, HUP
2016 Defenses
2016 Thesis Defense Schedule
- Christina Twyman – Saint Victor, MD “Overcoming Resistance to Immune Checkpoint Inhibitors in Metastatic Cancer”
Mentor: Andy Minn, MD, PhD
Friday, April 29 @ 2:30 PM
8030 Maloney Building, ITMAT Classroom - Elizabeth Bhoj, MD, PhD “Sequencing and Functional Characterization for Gene Discovery in Craniofacial Syndromes”
Mentor: Hakon Hakonarson, MD, PhD
Monday, May 2 @ 10 AM
8030 Maloney Building, ITMAT Classroom - Stephen Hunt, MD, PhD “Characterization of Treatment Effects and Immunobiology of Anti-Vascular Ultrasound”
Mentor: Chandra Sehgal, PhD
Monday, May 2 @ 2 PM8030 Maloney Building, ITMAT Classroom - Ann C. Gaffey, MD “Injectable shear-thinning hydrogels to deliver endothelial progenitor cells to improve cardiac function following ischemic injury”
Mentor: Pavan Atluri, MD
Thursday, May 5 @ 10 AM
BRB 251, Biomedical Research Building - Marisa Mizus, MD “Development, Optimization, and Validation of a Novel Urine Assay for Extracellular Vesicles”
Mentor: Emile R. Mohler III, MD
Thursday, May 5 @ 3 PM
8030 Maloney Building, ITMAT Classroom - Samantha Schon, MD “Histone Modifications in Normal and Abnormal Sperm”Mentor: Shelley Berger, PhD
Wednesday, May 25 @ 2:30 PM
8030 Maloney Building, ITMAT Classroom - Stephanie Chen, MD “Glutamate Imaging in Refractory Epilepsy”
Mentor: Kate Davis, MD, MSTR
Wednesday, June 15 @ 2 PM
8030 Maloney Building, ITMAT Classroom - Chijioke Egbujo, MD “The olfactory circuit as a translational probe for altered olfactory circuit in schizophrenia.”
Mentor: Chang-Gyu Hahn, MD, PhD
Thursday, June 16 @ 1:30 PM
8030 Maloney Building, ITMAT Classroom - Christopher M. Cielo, DO “Obstructive sleep apnea in children with craniofacial conditions”
Primary Mentor: Carole Marcus, MBBCh, Co-Mentor: Richard Schwab, MD
Tuesday, October 25 @ 11:30 am
8030 Maloney Building, ITMAT Classroom - Mariana Cooke, MD-PhD “Detection and characterization of genetic drivers in circulating tumor cells and in single cancer cells”
Research Mentor: Jay F. Dorsey, MD-PhD
December 16th, 2016 @ 1:00 pm
8030 Maloney Building, ITMAT Classroom

2023 Academic Calendar and Deadlines
| Date | Events |
|---|---|
| April 27 | Predoc TL1 Finance Orientation 10:00am, via Zoom |
| April 27 | Predoc TL1 Orientation 10:30am, via Zoom |
| April 27 | MSTR New Student Orientation 12:00pm – 1:30pm, 8030 Maloney |
| May 23 | Summer I Term Classes begin for current MSTR students |
| July 11 | Summer II Term Classes begin for MSTR students
Welcome Breakfast for New Students, 8:30-9:00am |
| July 13 | PSDP: 12:30 – 2 pm over lunch |
| August 5 | Full Summer term elective courses end. Note: End dates differ for students enrolled in MTR 6010 and 6020. |
| August 19 | MSTR Summer II Classes End |
| September 6 | MSTR Fall Courses Begin MTR 6000: Tues & Thurs, 8:30-10:00am *MTR 6000 begins Thurs, Sept 8th* MTR 6030: Tues & Thurs, 10:30-12:00pm |
| September 7 -17 2023
|
Student Thesis Proposal Presentations Primary mentor and thesis committee required to attend mentee presentation date. More information provided in MTR 6020 course. |
| TBD | Last Day to Drop/Add a course for Fall 2022 Term without financial obligation. |
| October 1 — November 12 | Fall Program Mentor Meetings to be held by Nov 12. Student, Primary Research Mentor and MSTR Program Mentor attend. |
| October TBD | MSTR Student Roundtable |
| TBD | Last Day to Drop/Add a course for Fall 2022 Term with 50% financial obligation and no notation on transcript. |
| October 11 — 12 | ITMAT Symposium Registration required. |
| November TBD | Last day to withdraw from a course — full financial obligation applies and transcript will show a ‘W’ |
| November TBD | Spring 2023 Registration Due to Program Office |
| November TBD | Penn IRB New Member Training Time, Location TBD |
| November 23 — 26 | Thanksgiving Holiday — No Classes |
| December 11 | Classes End |
| December 21 — January 3 | Winter Break |
2022 Academic Calendar and Deadlines
| Date | Events |
|---|---|
| April 19 | Course Orientation for MTR 6010 Review Writing 12:15pm – 1:00pm, 8030 Maloney |
| April 21 | Finance Orientation Sessions for KL2 and TL1 Scholars Multiple times, via Zoom |
| May 12 | MSTR New Student Orientation 12:00pm – 1:30pm, 8030 Maloney |
| May 23 | Summer I Term Classes begin for current MSTR students |
| June 29 | Summer I Term Classes end for current MSTR students |
| July 7 | Welcome Breakfast for New Students, 8:30-9:00am |
| July 7 | Summer II Term Classes begin for MSTR students MTR 6020: Tue & Thu, 9:00-10:30am MTR 6010: Tue & Thur, 10:45-12:15pm PSDP: 12:30 – 2 pm over lunch |
| August 5 | Full Summer term elective courses end. Note: End dates differ for students enrolled in MTR 601 and 602. |
| August 19 | MSTR Summer II Classes End |
| September 6 | MSTR Fall Courses Begin MTR 6000: Tues & Thurs, 8:30-10:00am *MTR 6000 begins Thurs, Sept 8th* MTR 6030: Tues & Thurs, 10:30-12:00pm |
| September 6 -16 2022
|
Student Thesis Proposal Presentations Primary mentor and thesis committee required to attend mentee presentation date. More information provided in MTR 6020 course. |
| September 13, 2022 | Last Day to Drop/Add a course for Fall 2022 Term without financial obligation. |
| October 1 — November 12 | Fall Program Mentor Meetings to be held by Nov 12. Student, Primary Research Mentor and MSTR Program Mentor attend. |
| October TBD | MSTR Student Roundtable |
| October 10, 2022 | Last Day to Drop/Add a course for Fall 2022 Term with 50% financial obligation and no notation on transcript. |
| October 11 — 12 | ITMAT Symposium Registration required. |
| November 7, 2022 | Last day to withdraw from a course — full financial obligation applies and transcript will show a ‘W’ |
| November 18 | Spring 2023 Registration Due to Program Office |
| November TBD | Penn IRB New Member Training Time, Location TBD |
| November 24 — 27 | Thanksgiving Holiday — No Classes |
| December 12 | Classes End |
| December 16 — January 11 | Winter Break |
2021 Academic Calendar and Deadlines
| Date | Events |
|---|---|
| March 25 | Orientation 9:00am via Zoom |
| April 1 — May 1 | Individual Team Mentor Meetings: Student, Primary Mentor(s), and MSTR Program Mentor required to attend. |
| May 24 | Summer I Term Classes begin for current MSTR students |
| June 30 | Summer I Term Classes end for current MSTR students |
| July 6 | Summer II Term Classes begin for MSTR students MTR 602: Tue & Thu, 9:00-10:30am MTR 601: Tue & Thur, 10:45-12:15pm |
| July 8 | Welcome Event for New Students |
| August 6 | Full Summer term elective courses end. Note: End dates differ for students enrolled in 601 and 602. |
| August 20 | MSTR Summer II Classes End |
| September 7 | MSTR Fall Courses Begin MTR 600: Tues & Thurs, 8:30-10:00am *MTR 600 begins Thurs, Sept 9th* MTR 603: Tues & Thurs, 10:30-12:00pm |
| September 7-17 2021
|
Student Thesis Proposal Presentations Primary mentor and thesis committee required to attend mentee presentation date. More information provided in MTR 602 course. |
| September TBD | Last Day to Drop/Add a course for Fall 2021 Term without financial obligation. |
| October 1 — November 12 | Fall Program Mentor Meetings to be held by Nov 12. Student, Primary Research Mentor and MSTR Program Mentor attend. |
| October 5 | MSTR Student Roundtable |
| October 11 — 12 | ITMAT Symposium Registration required. |
| November TBD | Last day to withdraw from a course — full financial obligation applies. |
| November 12 | Spring 2022 Registration Due to Program Office |
| November TBD | Penn IRB New Member Training Time, Location TBD |
| November 25 — 28 | Thanksgiving Holiday — No Classes |
| December 10 | Classes End |
| December 10 — January 12 | Winter Break |
2020 Academic Calendar and Deadlines
| Date | Events |
|---|---|
| March 2 | Orientation 3:00pm in the Collaboration Space, 15th Floor of PCAM |
| March 2 — June 1 | Individual Mentor Committee Meetings: Student, Primary Mentor(s), and MSTR Program Mentor required to attend. |
| May 26 | Summer I Term Classes begin for current MSTR students |
| July 1 | Summer I Term Classes end for current MSTR students |
| July 2 | Summer II Term Classes begin for MSTR students MTR 602: Tue & Thu, 9:00-10:30am MTR 601: Tue & Thur, 10:45-12:15pm *MTR 601 begins Tuesday, July 7th* |
| August 7 | Full Summer term elective courses end. Note: End dates differ for students enrolled in 601 and 602. |
| August 20 | MSTR Summer II Classes End |
| September 8 | MSTR Fall Courses Begin MTR 600: Tues & Thurs, 8:30-10:00am *MTR 600 begins Thurs, Sept 10th* MTR 603: Tues & Thurs, 2:00-3:15pm |
| September 8-18 2020
|
Student Thesis Proposal Presentations Primary mentor and thesis committee required to attend mentee presentation date. More information provided in MTR 602 course. |
| September 15 | Last Day to Drop/Add a course for Fall 2020 Term without financial obligation. |
| October 1 — November 13 | Fall Program Mentor Meetings to be held by Nov 13. Student and MSTR Program Mentor attend. |
| October 6 | MSTR Student Roundtable |
| October 12 — 13 | ITMAT Symposium Registration required. |
| November 9 | Last day to withdraw from a course — full financial obligation applies. |
| November 13 | Spring 2021 Registration Due to Program Office |
| November TBD | Penn IRB New Member Training Time, Location TBD |
| November 26 — 27 | Thanksgiving Holiday — No Classes |
| December 10 | Classes End |
| December 10 — January 13 | Winter Break |
2019 Academic Calendar and Deadlines
| Date | Events |
|---|---|
| March 18 | Orientation Part I 2:30 PM in 8030 Maloney, HUP |
| April — June 13 | Individual Mentor Committee Orientation Meetings: Student, Primary Mentor(s), and MSTR Program Mentor required to attend. |
| May 28 | Summer I Term Classes begin for current MSTR students |
| June 13 | Orientation Part II 3:30 PM in 15th Floor PCAM Collaboration Space |
| July 3 | Summer I Term Classes end for current MSTR students |
| July 9 | MSTR Summer II Classes Begin MTR 602: Tue & Thu, 9:00 — 10:30 am MTR 601: Tue & Thu, 10:45 — 12:15 pm |
| August 9 | Full Summer term elective courses end. Note: End dates differ for students enrolled in 601 and 602. |
| Aug 22 | MSTR Summer II Classes End |
| September 5 | MSTR Fall Courses Begin MTR 603: Tue & Thu, 2 — 3:15 pm MTR 600: Tue & Thu, 3:30 — 5:00 pm |
| September 10, 12, and 17, 2019 | Student Thesis Proposal Presentations, 9 — 11:15 am each day. Primary mentor and thesis committee required to attend mentee presentation date. More information provided in MTR 602 course. |
| September 10 | Last Day to Drop/Add a course for Fall 2019 Term without financial obligation. |
| September 19 | All Student PDC: Reimagining Data and it’s Potential 12-1:30 PM, 8030 Maloney, HUP |
| October 1 — November 15 | Fall Program Mentor Meetings to be held by Nov 15. Student and MSTR Program Mentor attend. |
| October 14-15 | 2019 ITMAT Symposium Registration required. |
| October 17 | First Year PDC: Presenting Your Best Self Online 12-1:30 PM, 8030 Maloney, HUP |
| October 31 | All Student PDC: Valuing Your Science 12-1:30 PM 8030 Maloney, HUP |
| November 4 | Last day to withdraw from a course — full financial obligation applies. |
| November 15 | Spring 2020 Registration Due to Program Office |
| November 19 | Penn IRB New Member Training *12:30-1:30pm in 8030 Maloney Bldg., HUP |
| November 21 | Second Year PDC: Communicating Your Science 12-1:30 PM, 8030 Maloney, HUP |
| November 28-29 | Thanksgiving Holiday — No Classes |
| December 5 | 2nd Year PDC: Foundations ofUnconscious Bias 12-2 PM Room TBD |
| December 9 | Classes End |
| December 10 | MSTR Student RoundTable |
| December 10 – January 14 | Winter Break |
2018 Academic Calendar and Deadlines
Deadlines and dates may differ from other programs and schools. This is particularly of note for elective courses. Dates are subject to change and vary from the Penn Academic Calendar: http://www.upenn.edu/almanac/3yearcal.html
| Date | Events |
|---|---|
| May 17 | New Student Orientation and Meet & Greet
Orientation at 3:30 pm |
| May 18 – July 6 | Individual Mentor Committee Orientation Meetings: Student, Primary Mentor(s), and MSTR Program Mentor required to attend. |
| July 10 | MSTR Summer II Classes Begin MTR 602: Tue & Thu, 8:30 — 10 am MTR 601: Tue & Thu, 10 — 11:30 am |
| Aug 23 | MSTR Summer II Classes End *does not apply to special term class MTR 602 |
| September 6 | MSTR Fall Courses Begin MTR 603: Tue & Thu, 2 — 3:15 pm MTR 600: Tue & Thu, 3:30 — 5:00 pm |
| September 11, 13, 18 | Student Thesis Proposal Presentations 9-11:15 am each date Students required to attend all dates, Primary mentor required to attend mentee presentation date. |
| September 17 | Last Day to Drop/Add a course for Fall 2018 Term without financial obligation. |
| October 15-16 | 2018 ITMAT Symposium Registration required. |
| October 3 — November 30 | Fall Program Mentor Meetings to be held by Nov 30. Student and MSTR Program Mentor attend. |
| November 10 | Last day to withdraw from a course — full financial obligation applies. |
| November (Date TBD) | IRB New Member Training |
| November 22 — 23 | Thanksgiving Holiday — No Classes |
| November 30 | Spring 2019 Registration due to program office |
| December (Date TBD) | MSTR Student Round Table |
| December 10 | Classes End |
| December 10 – January 13 | Winter Break |
2017 Academic Calendar and Deadlines
The important dates below are scheduled by ITMAT Education Programs. Some deadlines and other dates may be different in other programs and schools. This is particularly of note for elective course dates. Dates are subject to change and vary from the Penn Academic Calendar: http://www.upenn.edu/almanac/3yearcal.html
| Date | Events |
|---|---|
| May 18 | New Student Orientation and Meet & Greet
Orientation at 3:30 pm |
| May 18 – July 6 | Individual Mentor Committee Orientation Meetings: Student, Primary Mentor(s), and MSTR Program Mentor required to attend. |
| July 6 | MSTR Summer II Classes Begin |
| Aug 24 | MSTR Summer II Classes End *does not apply to special term class MTR 602 |
| September 7 | MSTR Fall Courses Begin |
| September 7, 12, 14, 19 | Student Thesis Proposal Presentations 9-11 am each date Students required to attend all dates, Primary mentor required to attend mentee presentation date. |
| September 18 | Last Day to Drop/Add a course for Fall 2017 Term without financial obligation. |
| October 17-18 | 2017 ITMAT Symposium Registration required. |
| October 17 – December 1 | Fall Program Mentor Meetings to be held by December 1. Student and MSTR Program Mentor attend. |
| November 24-27 | Thanksgiving Holiday |
| November (Date TBD) | IRB New Member Training |
| November 10 | Last day to Withdraw from a course |
| December 1 | Spring 2018 Registration due to program office |
| December (Date TBD) | MSTR Student Round Table |
| December 12 | Classes End |
| December 13 – January 10 | Winter Break |

