Professional Development Offerings from ITMAT Ed
ITMAT Education sponsors a number of professional development offerings ranging from workshops and short courses to cohort training and NIH funded study collaborations. A listing of all programming is below and will direct you to learn more about objectives, eligibility and current offerings.
ITMAT Ed Offerings
Clinical and Translational Research 101
Half-day introductory workshop for Residents and Fellows on conducting Clinical & Translational Research (CTR) at Penn.
Wednesday, November 13, 2019
8:00 am — 12:30 pm
15th Floor South Pavilion, Collaboration Space, Perelman Center for Advanced Medicine (PCAM)
3400 Civic Center Blvd, Philadelphia, PA, 19104
The following topics of interest for Residents and Fellows were covered:
- An Overview of CTR at Penn
- Training Opportunities
- Resources to Support Clinical Research Execution
- Approaches to Writing a Research Proposal & Putting it all Together
Questions?
Contact Jessica German.
2019 Presentations and Resources
Slides:
- Overview and Training Opportunities for Clinical and Translational Research
- How to use REDCap for Your Research Project
- Where is Clinical Research Done — the Center for Human Phenomic Science (CHPS)
- How to Leverage Available Support Services for Clinical Research
- How to Access Data — Penn Information Systems (IT)
- Navigating the IRB
- Approaches to Writing a Research Proposal & Putting it All Together
Resources:
- IRB Handout
- Become an ITMAT member here
- Penn Center for Health Care Innovation
- Looking for Biostats support? Contact Andy Cucchiara, PhD.
2018 Presentations and Resources
- Overview of CTR at CHOP
- Overview of CTR at Penn
- Training Opportunities in CTR
- Biostatistical & Data Management Support
- Penn Data Analytics Center (DAC)
- CHOP Clinical Research Support Office (CRSO)
- Perelman School of Medicine Office of Clinical Research (OCR)
- Writing Up Your Results
- Putting it all Together
Other Resource Links:
2017 Presentation Recordings & Speaker Information
Session Recordings & Speaker or Office Contact Information
- Overview of CTR at CHOP
- Overview of CTR at Penn
- Opportunities to Pursue a CTR Career at Penn
- Precision Medicine Overview
- Sara Cherry, PhD and Abigail Berman, MD, MSCE — Penn Center for Precision Medicine
- Biostatistics & Data Management Support
- Data Analytics Center Recording
- CHOP Clinical Research Support Office
- Perelman School of Medicine Office of Clinical Research
- Planning your CTR Projects
Other Resource Links:
2016 Presentation Recordings with Slides
2019 Junior Investigators' Symposium

The Institute for Translational Medicine and Therapeutics (ITMAT) is pleased to host a one-day symposium for junior investigators (physician fellows, postdoctoral fellows, and junior faculty conducting research) focused on successful strategies for a career in clinical and translational science. Attendees will gain valuable career insights through presentations, panel discussions, and time for networking.
2019 Junior Investigators’ Symposium:
Tuesday, April 16, 2019
8:30AM to 4:00PM
Smilow Center for Translational Research
Slides & Session Recordings:
Session slides, where available, are linked below. Session recordings are available here.
Session Resources:
- Best Practices for Effective Mentor-Mentee Relationships
- Grant Resources
- Building Your Research Team
Agenda:
Time |
Session |
Location |
||
|---|---|---|---|---|
| 8:00-8:30AM | Registration and Continental Breakfast | SCTR Commons | ||
| 8:30-8:45AM | Welcome
Jonathan Epstein, MD, Executive Vice Dean and Chief Scientific Officer, Perelman School of Medicine, University of Pennsylvania Orientation Emma A. Meagher, MD |
Rubenstein Auditorium | ||
| 8:45-10:15AM | Unraveling the Mystery of Academic Tracks
Moderator: Kathy Shaw, MD, MSCE Panel: Tiffani Johnson, MD, MSc; Marina Cuchel MD, PhD, MSTR; Andy Minn, MD, PhD |
Rubenstein Auditorium | ||
| 10:15-11:00AM | Protecting Your Discoveries
Viviane Martin, PhD |
Rubenstein Auditorium | ||
| 11:00-11:40AM | Core Tenets of Effective Mentee-Mentor Relationships
Emma A. Meagher, MD |
Rubenstein Auditorium | ||
| 11:40- 12:00PM |
Lunch — Please fill your lunch box and head to your assigned session. | SCTR Commons | ||
| 12:00-12:40PM | Speed Mentoring | Pursuing Careers in Industry — Panel & Lunch
Moderator: Philippe Szapary, MD, MSCE |
SCTR Commons | SCTR 10-146 AB |
| 12:45-1:25PM | Pursuing Careers in Industry — Panel & Lunch
Moderator: Philippe Szapary, MD, MSCE |
Speed Mentoring | SCTR 10-146 AB | SCTR Commons |
| 1:35-2:45PM | Writing a Successful Career Development Grant (K Award)
Moderator: Jonathan Katz, MD Panel: Erum Hartung, MD, MSTR and Susan Furth, MD, PhD; Jeffrey Thompson, MD, MSTR and Steven Albelda, MD; Kiran Musunuru, MD, PhD, MPH |
Rubenstein Auditorium | ||
| Writing a Successful NIH R Grant
Moderator: David Mankoff, MD, PhD Panel: Despina Kontos, PhD; David Fajgenbaum, MD, MBA, MSc; Kelly Allison, PhD; Matthew Weitzman, PhD |
SCTR 8-146 AB | |||
| 2:50-3:55PM | Establishing Your Clinical Research Program & Building Your Team
Moderator: Joel Gelfand, MD, MSCE Panel: Mark Neuman, MD, MSc; Alexis Ogdie-Beatty, MD, MSCE; Anuja Dokras, MD, PhD; Elizabeth Steider, MBE |
SCTR 8-146 AB | ||
| Establishing Your Basic Research Program & Building Your Team
Moderator: Benjamin Voight, PhD Panel: Elizabeth Grice, PhD; Elizabeth Heller, PhD; Eric Joyce, PhD |
Rubenstein Auditorium | |||
Location:
Smilow Center for Translational Research
Rubenstein Auditorium and Smilow Commons
3400 Civic Center Blvd
Philadelphia, PA 19104
Directions: For directions to the Rubenstein Auditorium on the first floor of Smilow Center for Translational Research (SCTR) click here.
For Panel Sessions held on floor 8 or 10 SCTR in 146AB conference rooms: Take the Smilow elevators to the designated floor, enter through the glass doors, turn right, and follow the hall as far right as possible to the 146AB conference rooms. Signs will be posted as well.
Speed Mentoring: Attendees will have the opportunity to spend time one-on-one with seasoned faculty members.
Registration: There is no cost to attend, but registration is required. Registration is now closed.
Questions can be directed to Jessica German.
Past Symposia
2017 Junior Investigators' Symposium

The Institute for Translational Medicine and Therapeutics (ITMAT) is pleased to host a one-day symposium for junior investigators (physician fellows, postdoctoral fellows, and junior faculty conducting research) focused on successful strategies for a career in clinical and translational science. Attendees will gain valuable career insights through presentations, panel discussions, and time for networking.
2017 Junior Investigators’ Symposium:
Tuesday, March 21, 2017
7:30AM to 5:00PM
Smilow Center for Translational Research
Slides & Session Recordings:
Session slides, where available, are linked below. Session recordings are available here.
Agenda:
Time |
Session |
Location |
|---|---|---|
| 7:30-8:00AM | Registration and Continental Breakfast | Smilow Commons |
| 8:00-8:15AM | Welcome J. Larry Jameson, MD, PhD, Dean, Perelman School of Medicine, University of PennsylvaniaOrientationDennis Durbin, MD, MSCE and Emma Meagher, MD |
Rubenstein Auditorium |
| 8:15-9:45AM | Unraveling the Mystery of Academic Tracks
Moderator: Kathy Shaw, MD, MSCE Panel: Angie Ellison, MD, MSc; Erum Hartung, MD, MTR; Eric Joyce, PhD; Ben Voight, PhD |
Rubenstein Auditorium |
| 9:45-10:00AM | Coffee Break | Smilow Commons |
| 10:00-11:10AM | Core Tenets of Effective Mentee-Mentor Relationships
Moderators: Emma Meagher, MD and Dennis Durbin, MD, MSCE |
Rubenstein Auditorium |
| 11:15-12:30PM | Writing a Career Development/K Award
Moderator: Jonathan Katz, MD Panel: Sandra Amaral, MD, MHS & Sue Furth, MD, PhD; Amanda Zacharias, PhD & John Murray, PhD |
SCTR 8-146AB |
| Writing a Successful NIH R Grant
Moderator: Garret FitzGerald, MD Panel: Casey Brown, PhD; Yael Mosse, MD; Matthew Weitzman, PhD |
Rubenstein Auditorium | |
| 12:30-1:15PM | Lunch & Networking | Smilow Commons |
| 1:20-2:15PM | Navigating Research Resources: Clinical Session
Moderators: Emma Meagher, MD and Dennis Durbin, MD, MSCE |
SCTR 8-146AB |
| Navigating Research Resources: Lab Session
Moderators: Celeste Simon, PhD and Harry Ischiropoulos, PhD |
SCTR 9-146AB | |
| 2:20-3:45PM | Speed Mentoring | Smilow Commons |
| Research Vendor Forum | ||
| Coffee Break | ||
| 3:45-4:30PM | Building Your Brand and Online Presence
Rachel Locke, PhD |
Rubenstein Auditorium |
| 4:30-5:00PM | Symposium Keynote
Jonathan Epstein, MD, Executive Vice Dean and Chief Scientific Officer, Perelman School of Medicine, University of Pennsylvania |
Rubenstein Auditorium |
| 5:00PM | Networking Reception | Smilow Commons |
Location:
Smilow Center for Translational Research
Rubenstein Auditorium and Smilow Commons
3400 Civic Center Blvd
Philadelphia, PA 19104
Directions: For directions to sign in and the Rubenstein Auditorium on the first floor of Smilow Center for Translational Research (SCTR) click here.
For Panel Sessions held on floor 8 or 10 SCTR in 146 AB conference rooms: Take Smilow elevators to the designated floor, enter through glass doors, turn right, and follow the hall as far right as possible to the 146AB conference rooms. Signs will be posted as well.
Speed Mentoring: Attendees will have the opportunity to spend time one-on-one with seasoned faculty members.
Registration: There is no cost to attend, but registration is required. Registration is now closed.
Questions can be directed to Anna Greene
Professional Skills Development Program
Formally launched in 2016, the Professional Skills Development Program (PSDP) is designed to expose early career scholars and trainees in clinical translational science to the wide range of environmental and individual factors that contribute to a successful career. The primary objectives of the PSDP are to
- support the unique professional development needs of CTSA scholars at varying critical stages from medical school trainees to assistant professors.
- create a community experience for cohorts that encourages and fosters meaningful interaction with peers and program leadership.
The PSDP seeks to meet these objectives through interactive presentations, directed experiences, and facilitated discussions. Participants are introduced to, and gain practice with, a wide range of skills and aptitudes demonstrated by effective leaders and professionals in academia, entrepreneurship, and professional and personal development. Facilitators include ITMAT Education faculty leadership as well as topic experts from Penn, Children’s Hospital of Philadelphia (CHOP), the larger University of Pennsylvania community, industry, and other CTSA institutions.
The Multiple Layers of the PSDP
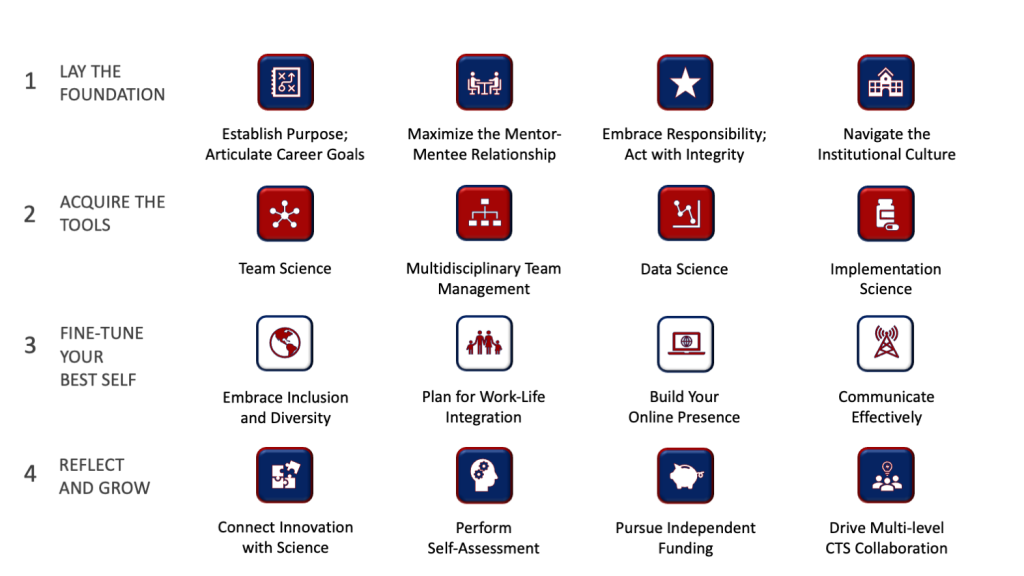
As a result, participants are able to identify resources, concepts, skills, and behaviors relevant to their work as clinicians, scientists, leaders and entrepreneurs. Following self-reflection, observation of examples in action, practical implementation and peer exchange, participants will be well positioned to implement their personal style in their daily work, no matter their current role or future professional pathway taken.
Sessions are reserved for MSTR and MSRS students, predoctoral TL1 and postdoctoral TL1 trainees, and KL2 scholars.
Workshops on Statistical Software
ITMAT Education provides statistical software workshops to the ITMAT Education learning community which includes students, alumni, and ITMAT members.
Stata Workshops
Dr. Andrew Cucchiara, PhD, leads Stata workshops for students to learn basic Stata programming and actively apply the software to existing data sets. The workshops present the content:
- In short video tutorials presenting core concepts and processes
- With hands-on learning opportunities through existing data sets
- In an online environment where learners engage in small groups
Bioinformatics Workshops
Dr. Benjamin Voight, PhD, leads bioinformatics software workshops to the ITMAT Education learning community which includes students, alumni, and ITMAT members. These workshops include:
- Python programming: bioinformatics analyses often require that students execute programs on the UNIX command line. If the data are large enough, this may require use of cluster computing to perform the task and manage the analysis. Moreover, there are specific tools available in the Python working environment that are extremely useful, e.g., scikit-learn for machine learning.
- R data science visualization: visualization of data and creating ‘display items of merit’ that are publication quality. Providing this workshop during the data exploration and analysis phase will be especially valuable. Having a code base for visualizations on processed data sets will help us meet reproducibility standards and save time. We will develop a workshop which introduces participants to ggplot2 / tidyverse, both very relevant R packages for data science and visualization. We also plan to deploy content into a library of online learning modules, which students could have ongoing access to and could self-teach.
- Technical reproducibility in R: with the assumption that analysis embedded in computer code should be technically reproducible, this workshop plans to introduce trainees to a range of tools and websites that provide this technical capability (github, Jupyter notebooks, R markdown, shiny apps, etc) and demonstrate how they can be used.
The workshops will present the content in short video tutorials presenting core concepts and processes, with hands-on learning opportunity through existing data sets, in an online learning environment where learners engage in small groups.
Learners will have the option to enroll in MTR 535 Intro to Bioinformatics in Spring 2021.
ITMAT Ed Collaborations
- KL2 CTSA Visiting Professorship Program
- University of Pittsburgh — Building Up a Diverse Pipeline for the Biomedical Research Workforce
- University of Pittsburgh — R21 Customized Career Development Platform (CCDP) Study
- UE5 Short Course: Cell & Gene Therapy Toolkit for Junior Faculty
- Advanced Therapies Intensive
Organized via a working group out of the Center for Leading Innovation & Collaboration (CLIC), this program provides an opportunity for KL2 Scholars to virtually visit a CTSA institution, give a national talk and meet with faculty from the CTSA institution. The first cohort of KL2 scholars visits will begin Fall 2020. Dr. Emma Meagher, Director of ITMAT Education, serves on this CLIC Working Group Leadership Committee.
Aims to jump-start the careers of underrepresented junior investigators by providing them with the mentoring, skills, and knowledge needed for successful research careers. Dr. Emma Meagher, Director of ITMAT Education, serves as the site chamption for Penn.
A multi-site trial under the leadership of PIs Dr. Doris Rubio and Dr. Cecilia Patino-Sutton to understand the effectiveness of different types of individualized development plans (IDPs). Dr. Emma Meagher, Director of ITMAT Education, serves as a Co-Investigator for this study.
UE5 Short Course: Cell & Gene Therapy Toolkit for Junior Faculty
As a collaborative initiative between the Institute of Translational Medicine and Therapeutics (ITMAT), the Abramson Cancer Center, and Oncology at the Children’s Hospital of Philadelphia (CHOP), the University of Pennsylvania (Penn) is honored to be one of four institutions selected to host a short course education opportunity for junior faculty within the NCI Awardee Skills Development Consortium (NASDC).
Building on a history of success in both cell and gene therapies at Penn and CHOP, our UE5 short course focuses on providing NCI young investigator awardees with a comprehensive background in the latest scientific, translational, clinical, regulatory, and commercial advances of cell and gene therapy so that they are equipped with the knowledge to translate research findings into successful cancer therapies.
The course integrates and expands on an existing foundation of related educational programs and experienced faculty-educators at Penn and CHOP. It covers in modular format the foundations, preclinical tools, and protocols for biomanufacturing as well as intellectual property, regulatory compliance, clinical trial design, and systemic challenges in collaborative science.
Visit the NASDC consortium homepage to learn more about course dates and the application process.
Penn UE5 Leadership & Contributing Faculty
Contributing faculty to the Penn UE5 are pioneers in the field of cell and gene therapy across the translational spectrum, from discovery to the first FDA approvals of both cell and gene therapy products, and have been responsible for major advances at Penn, CHOP, the Abramson Cancer Center and globally. In addition to basic scientists, the faculty includes experts and specialists in areas key to translating immunobiology into clinical trials, including specialists in translational and clinical research, biomarker characterization for cell and gene therapy, regulatory science, and commercial collaboration.
Contact
General inquiries regarding the Penn UE5 may be sent to Cauleen Noël, Staff Lead for the Abramson Cancer Center’s Research Education and Training component. All inquiries regarding the application and selection process may be submitted directly to the NASDC Consortium.
PIs and Course Directors
Elizabeth O. Hexner, MD, MSTR
Dr. Hexner is a hematologist with an interest in gene modified cellular therapy, hematologic malignancies, and graduate education. She serves as Medical Director for the Center for Cellular Immunotherapies, Director of the Penn MPN program, and Associate Director for the Masters in Translational Research program at the University of Pennsylvania.
David A. Mankoff, MD, PhD
Dr. Mankoff’s contribution is within context of his role as the Associate Director of Education and Training for the Abramson Cancer Center (ACC) to help direct and coordinate education resources to support the short course. He is highly recognized for his expertise in the area of cell and gene therapy as both a translational cancer-focused investigator and leader in a number of Penn education programs directed toward postdoctoral fellows, GME trainees, and junior faculty.
Stephan A. Grupp, MD, PhD
Dr. Grupp is a world authority on the development and clinical application of CAR T cells and other engineered cell therapies, having led the registration trial for Kymriah, the first CAR T product.
Andrew Fesnak, MD
Dr. Fesnak is the Director of Manufacturing in the Clinical Cell and Vaccine Production Facility as well as Director of Regulatory Education programs at Penn, and is an educator in the field of cell therapy.
UPenn-KHP Advanced Therapies Intensive
Advanced Therapies Intensive – September 2022
The Perelman School of Medicine at the University of Pennsylvania in partnership with King’s Health Partners and King’s College London is offering, for the second time, a one-week transatlantic educational experience for trainees focused on the development of novel therapeutics. The intensive is led by Emma Meagher, MD from Penn, Anne Greenough, MD from KCL, and Francesco Dazzi, MD, PhD from KCL. The week will include presentations with expert faculty and small group learning activities.
Dates and Location
- Monday, September 12 – Friday, September 16, 2022
- 8-12pm EST; 1-5pm BST (Except Friday, 9/16 – time extends by 30 mins)
- Online, Asynchronous and Synchronous
About the Intensive
This one-week global intensive provides MD and PhD participants, including PhD Candidates, from the University of Pennsylvania and King’s Health Partners with an overview of developing novel therapeutics. Topics include stem cells and regenerative medicine, clinical trial ethics, cell therapies and gene editing, a roadmap to initiating translation, and a roadmap to commercialization. Participants are introduced to these topics through asynchronous and synchronous learning environments. The content of the intensive includes seminars, case studies, group projects, and journal article-reviews.
Upon completion of the intensive, the participants should be able to:
- Recognize the cutting-edge science underpinning the development of novel therapeutics
- Assess the complex regulatory landscape for development of cells for medical treatment
- Apply approaches to the ethical design and implementation of advanced therapy clinical trials
- Determine the importance of producing credible business plans for the application of cellular therapies
Eligibility
- MDs, PhDs, and PhD candidates (who have progressed in their studies beyond their prelim examination) at Penn, CHOP, KHP, KCL who are interested in developing novel therapies
- Participants must commit to full attendance and complete all assignments, inclusive of pre-readings, in advance of synchronous sessions
- Eight US and eight UK participants will be selected
Apply by May 13, 2022
Materials required for a complete application include an application form which requires the submission of a CV and a recorded 5-minute elevator pitch briefly introducing yourself, describing your background, why you are interested in this intensive, and how you believe it will further your learning and career goals. See the attached Guide to Recording and Submitting Your Pitch in Zoom.
Click here to submit an online application.
There is no cost associated with this program.
Contact
For questions related to the intensive, contact Jessica German at jbgerman@upenn.edu
Intensive Spotlights
2021 KHP News Articles:
- Students praise Advanced Therapies Intensive Course as ‘a rare opportunity’
- Further praise for the Advanced Therapies Intensive Course
Photos of the Inaugural Cohort
Other Professional Development Resources
Academic Entrepreneurship for Medical and Health Scientists eBook
The Children’s Hospital of Philadelphia (CHOP) Research Institute partnered with the University of Pennsylvania’s (Penn) Institute for Translational Medicine and Therapeutics (ITMAT) to seed fund a grassroots effort of editors, subject matter experts, and translational research students to create a free Open Education Resource that seeks to support and grow a new academic research community of Boundary Spanners. This e-book is a repository of tools, wisdom and best practice that seeks to democratize innovation regardless of where the academic researcher resides. Drs. Nalaka Gooneratne, Flaura Winston and Ms. Rachel McGarrigle contributed as editors.
I2SEED
An Interdisciplinary Training Program in Entrepreneurship and Translational Research for Alzheimer’s Disease and AD Related Disorders
Program Overview
I2SEED is an entrepreneurship focused education program which provides training to help develop and commercialize diagnostic, supportive or therapeutic interventions for Alzheimer’s Disease and AD Related Disorders (ADRD).
Participants who complete this program will gain the skills necessary to successfully submit a highly competitive NIA SBIR or STTR grant. We also offer sustained engagement and support to assist with a resubmission, if necessary. The ultimate goal of this program is to maximize the likelihood that participants will be able to obtain seed funding through a combination of SBIR/STTR grant(s) and investor funding so that trainees can successfully transition to a funded career path that focuses on moving ADRD research from the bench to the bedside via a successful business plan.
This program is designed for pre- and post-doctoral trainees interested in exploring opportunities to commercialize ADRD diagnostics/therapeutics/devices
- Pre-doctoral trainees must be enrolled in a Master’s level or higher degree program
- Post-doctoral trainees must have completed a PhD, MD, DDS, or comparable doctoral degree from an accredited domestic or foreign academic institution
Five to ten trainees will be enrolled in the program each year and expected to participate in the program for two years (approximately 2-8 hours per week). This program is funded by the NIA and available at no cost to trainees.
Opportunities for Trainees
- Targeted education on biomedical entrepreneurship in a small group setting
- Networking opportunities
- Experiential learning with start-ups and commercial ventures
- Long term mentorship
- Travel funds for relevant conferences
- STTR/SBIR grant writing assistance
- Assistance developing and refining an investor pitch deck
Curriculum
This curriculum was designed to ensure that the projects designed and implemented by trainees take into account the myriad of factors relevant to the complex characteristics of biomedical entrepreneurship and important considerations of developing preclinical and late-phase ADRD trials. The core curriculum will include the following elements:
Integrated Core Curriculum
Didactics will begin as Topic Overview Sessions. These will consist of mini interactive overviews of the core domains of:
- Ideation
- Intellectual property and regulation
- Finance
- Team building and scientific cooperation
- ADRD Specific Topics
Each session will be approximately 30-45 min in length. Topic Overview Sessions will occur via video conference and be scheduled based on trainees availability. At the conclusion of each lecture trainees will be asked to rate their interest in each topic covered, this rating will be used to design the boot camp curriculum.
The diverse curriculum will also ensure that trainees are exposed to a broad range of skill sets required for ADRD biologic and therapeutic development through entrepreneurial and commercialization pathways.
Mini Bootcamp
The Bootcamp for the 2023-2024 class will be held: September – October 2023
After the Topic Overview Sessions, we will hold mini boot camp sessions on each topic.
The bootcamp is intended as an intensive introduction to more complex concepts related to entrepreneurship, product development, commercialization, and the unique demands of developing novel ADRD-related technologies and therapeutics. The feedback from Topic Information Sessions will be used to design a boot camp curriculum that is valuable to trainees. We anticipate 2-8 hours of lectures per week, interspersed with team-building activities such as social events, and networking opportunities to engage with other faculty.
NIH Grant Writing Workshop
Once the bootcamp is completed we will transition to focus on SBIR/STTR grant writing. Workshops will be hosted by Dr. Gooneratne via videoconference. These grant workshops will be held weekly and may occur more frequently when the group approaches grant submission deadlines.
Workshops will guide trainees through the complex process of preparing and submitting a SBIR/STTR grant and will include:
- Instruction on required SBIR/STTR components: i.e., NIH biosketch, specific aims, research strategy, resources and human subjects; and key elements necessary for ADRD research
- Review and discussion of successful ADRD SBIR/STTR applications
- Guided writing/timeline: trainees will first prepare their Specific Aims, to be discussed by all trainees and faculty in the workshop.
- Continuous Feedback: trainees will present their writing progress to faculty once per month; including a discussion of timeline, milestones, and progress to further refine project/grant.
- Mock Review: approximately 4 weeks before submission trainees will submit for a mock study section by mentors and other trainees that will provide insight into grant review process and allow for further refinement of grant
- Sustained engagement in the following year of the program to assist with the SBIR/STTR A1 grant resubmission, if necessary
ADRD Research Seminars
Approximately once every two months, we will invite guest lecturers to present at a State-of-the-Art Research seminar on ADRD. Trainees will hear from the brightest minds in the field from across multiple institutions who will be presenting on recent developments in the three core areas of disease biology, data sciences and drug discovery related to ADRD.
These talks will include discussions of novel neuroimaging methods, data analytic tools, animal models, and other recent developments to ensure that trainees are exposed to cutting edge research that may impact their application.
This seminar will also serve as a helpful networking opportunity for the trainees to connect with top researchers in the field.
Research Experiences and Mentoring
Fundamental to this program is its innovative mentorship framework that extends beyond a research mentor and business mentor to include a clinical mentor with experience in ADRD to provide a unique perspective that is central to commercial success. Trainees will be encouraged to maintain prior connections as mentor candidates. The I2SEED program will also connect trainees to mentors with expertise in their area of interest. The curriculum includes regular meetings with the trainee and their mentors to provide feedback and track progress.
This will also include experiential learning opportunities such as clinical shadowing, industry externships and networking to facilitate patient and caregiver stakeholder interactions for the trainee as appropriate.
Academic Entrepreneurship Textbook
Academic Entrepreneurship for Medical and Health Scientists eBook will serve as the content source for much of the didactic material. It is a free open-education resource, which was developed by Dr. Gooneratne and several other colleagues to fill a training gap in the commercialization of biomedical advances.
The book can be accessed on pubpub here: https://academicentrepreneurship.pubpub.org/
Program Leadership
 Nalaka Gooneratne, MD, MSci
Nalaka Gooneratne, MD, MSci
Program Director
Dr. Gooneratne is an Associate Professor at the University of Pennsylvania and the Associate Director for the Master’s of Science in Translational Research at the University of Pennsylvania. He is a board-certified sleep disorders physician. His research includes a multi-site NIA intervention study on sleep apnea in Mild Cognitive Impairment and he has been the PI on two NIH Small Business Grants.
Profile Page
 Katie Reuther, PhD, MBA
Katie Reuther, PhD, MBA
Co-Program Director
Dr. Reuther is the Executive Director of the Penn Center for Health-Devices and Technology (Penn Health-Tech) and on the faculty of the School of Engineering and Applied Science as Practice Associate Professor in Bioengineering. She also has experience as a co-founder of Syntegrity Biomedical, an orthopedic medical device start-up.
Profile Page
Apply Now
Applications will be evaluated on a rolling basis so we encourage you to apply early for early consideration.
- Pre-doctoral applicants must be enrolled in a Master’s level or higher graduate degree program and have at least a bachelor’s degree
- Post-doctoral applicants must have completed a PhD, MD, DDS, or comparable doctoral degree
- Applicants must be from an accredited institution
Application Process
Applicants must submit a full application which contains two parts:
1. Online I2SEED Application
Consisting of an electronic application form and the following documents:
- Personal Information
- CV/Biosketch
- Supplemental Questions
To gain access to the I2SEED online application and additional instructions please contact Jennifer Morris, MS, Education Program Director.
2. Letter of Support
Detailed information/instructions about the letter of support will be provided once interested applications complete the online application.
Application Support
One-to-one application support is available! Just contact us to discuss your potential application or with any questions about the program
Contact
Administration
For questions related to the I2SEED Training Program, please contact Jennifer Morris.
Nalaka Gooneratne, MD, MSci | I2SEED Program Director | ngoonera@pennmedicine.upenn.edu
Jennifer Morris, MS | I2SEED Program Education Director | jennifer.morris3@pennmedicine.upenn.edu

